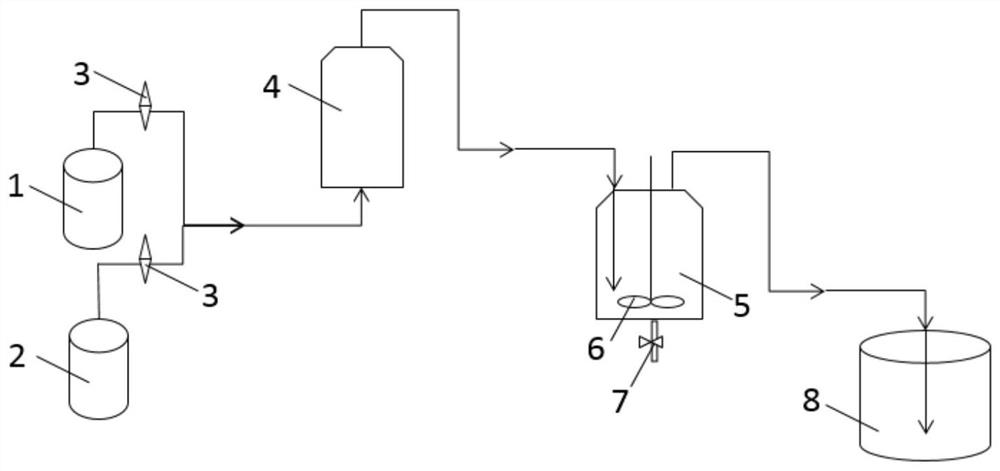
Perfluoropolyether end group fluorination method
A technology of perfluoropolyether acyl fluoride and perfluoropolyether, applied in the field of perfluoropolyether end group fluorination, can solve the problems of product loss, molecular structure, average molecular weight viscosity change, perfluoropolyether self-polymerization, etc. Achieve the effect of less product loss and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Build the above-mentioned fluorination device, add 500g of perfluoropolyether acid fluoride to the reactor at 20°C, its average molecular weight is 1120g / mol, vacuumize the reactor to -0.1MPa, turn on the stirrer, and turn on the ultraviolet high pressure Mercury lamp (400W), open the valves of the nitrogen and fluorine cylinders, monitor and adjust the flow rate to 20mL / min for nitrogen and 80mL / min for fluorine with a gas flowmeter, and activate the mixture of nitrogen and fluorine in a photoactivated airtight container for 80s Finally, open the valve between the light-activated airtight container and the reactor, monitor it with a gas flowmeter and feed it into the reactor at a rate of 100mL / min for reaction. Monitor the progress of the reaction. After the reaction was finished (after 6.5 hours of continuous ventilating), nitrogen was passed into the reactor for purging, and after cooling, the discharge valve at the bottom of the reactor was opened, and the material ...
Embodiment 2
[0040] Build the above-mentioned fluorination device, add 500g of perfluoropolyether acid fluoride to the reactor at 20°C, its average molecular weight is 1480g / mol, vacuumize the reactor to -0.1MPa, turn on the agitator, turn on the ultraviolet high pressure Mercury lamp (400W), open the valves of the nitrogen and fluorine cylinders, monitor and adjust the flow rate to 20mL / min for nitrogen and 80mL / min for fluorine with a gas flowmeter, and activate the mixture of nitrogen and fluorine in a photoactivated airtight container for 80s Finally, open the valve between the light-activated airtight container and the reactor, monitor it with a gas flowmeter and feed it into the reactor at a rate of 100mL / min for reaction. Monitor the progress of the reaction. After the reaction finished (after 4.5 hours of continuous ventilating), feed nitrogen into the reactor for purging, open the discharge valve at the bottom of the reactor after cooling, and discharge to obtain 499g of product, ...
Embodiment 3
[0042] Build the above-mentioned fluorination device, add 500g of perfluoropolyether acid fluoride to the reactor at 25°C, with an average molecular weight of 550g / mol, vacuumize the reactor to -0.1MPa, turn on the agitator, and turn on the ultraviolet high pressure Mercury lamp (400W), open the valves of the nitrogen and fluorine cylinders, monitor and adjust the flow rate to 20mL / min for nitrogen and 80mL / min for fluorine with a gas flowmeter, and activate the mixture of nitrogen and fluorine in a photoactivated airtight container for 80s Finally, open the valve between the light-activated airtight container and the reactor, monitor it with a gas flowmeter and feed it into the reactor at a rate of 100mL / min for reaction. Monitor the progress of the reaction. After the reaction was finished (after 13 hours of continuous ventilation), nitrogen was passed into the reactor for purging, and after cooling, the discharge valve at the bottom of the reactor was opened, and the materi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com