Application of galangin and derivatives thereof in preparation of drugs for preventing and treating nervous system diseases
A neurological disease, galangin technology, applied in the field of neurological disease drugs, can solve the problems of not finding galangin or any derivative thereof, such as anti-epileptic activity, analgesia, and changing neuronal excitability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
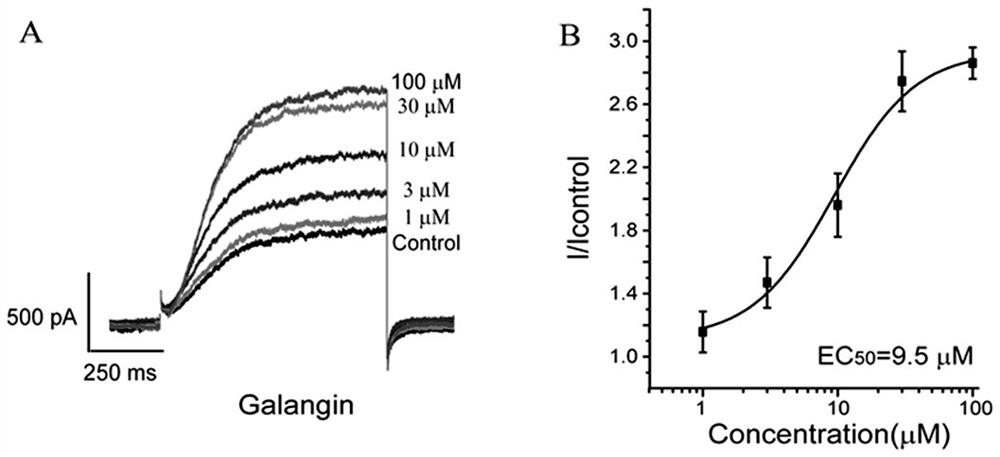
[0025] electrophysiological patch clamp technique
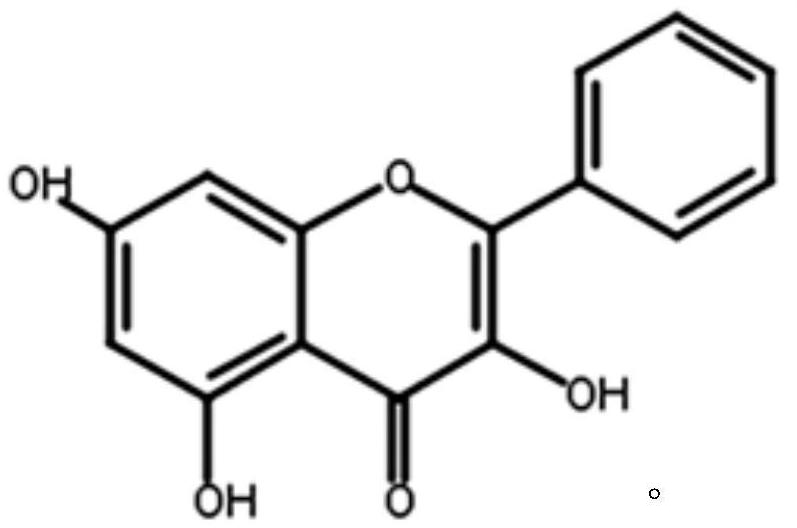
[0026] (1) Test compound: galangin
[0027] (2) Test method: Cultivation of Chinese hamster ovary cells (CHO): CHO cells stably transfected with KCNQ2 / Q3 channels were cultured in a medium containing 10% fetal bovine serum, 500ug / mL hygromycin and G418, 100U / mL penicillin and strepto Mycin B in DMEM culture solution, trypsinized and passaged. Cells were plated on 12mm round coverslips and cultured in 24-well plates.
[0028] Patch clamp technique to record cell membrane current: HEKA-EPC10 was used as patch clamp amplifier. Amphotericin B (final concentration 0.1-0.2mg / mL) was applied to the electrodes for punch-hole patch-clamp recording. After the microelectrode is polished, fill the inner liquid of the electrode, and control the resistance value at 2-4MΩ. The electrode inner solution used when recording CHO cells is (mM): KCl 160, HEPES 5, MgCl 2 3. CaCl 2 1, EGTA3, adjust pH to 7.4 with KOH; extracellular fluid co...
Embodiment 2
[0032] Pharmacodynamic experiment of galangin
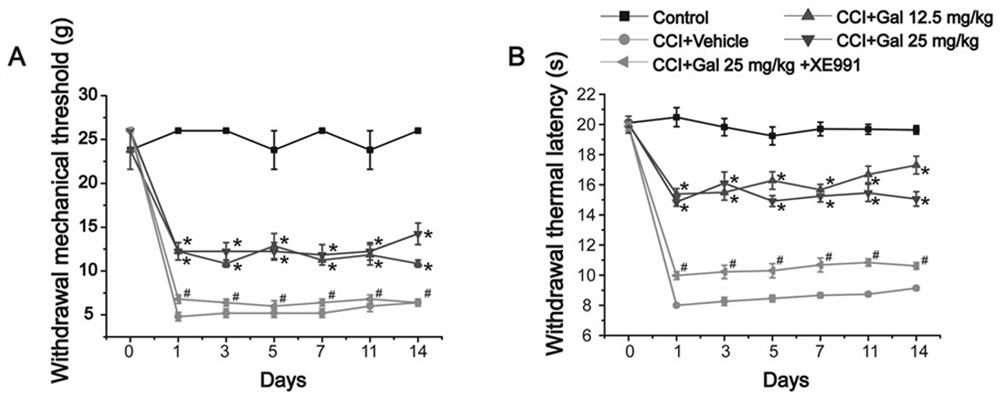
[0033] Pentetrazole PTZ-induced seizure model:
[0034]Experimental drug: 2% Tween 20 suspension of galangin (Galangin), and 2% Tween 20 suspension of retigabine in the control group. Experimental method: Male mice in each group were injected intraperitoneally with 80 mg / kg of pentylenetetrazole to induce epilepsy, and the latency of seizures, duration of seizures, number of seizures and grades of seizures were observed within 90 minutes. Intraperitoneal injection of galangin, the dosage was 12.5mg / kg, 25mg / kg, 50mg / kg respectively; the dosage of retigabine (RTG) in the control group was 15mg / kg. The experimental results are shown in Table 1, galangin can significantly increase the latency of pentylenetetrazol-induced epilepsy in a dose-dependent manner, and significantly reduce the duration of epilepsy, the number of seizures and the grade of seizures. In summary, galangin has significant antiepileptic effect.
[0035] Table ...
Embodiment 3
[0040] Tablets are prepared according to methods known in the art, each containing the following ingredients:
[0041] Galangin 50mg, lactose 70mg, magnesium stearate 3mg, polyvinylpyrrolidone 130mg.
[0042] Capsules are prepared according to methods known in the art, and each capsule contains the following ingredients:
[0043] Galangin 50mg, lactose 70mg, corn starch 25mg, magnesium stearate 1mg, polyvinylpyrrolidone 130mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com