Preparation method of 5, 6-dimethylbenzimidazole
A technology of dimethylbenzimidazole and dimethylphenyl, which is applied in the field of preparation of 5,6-dimethylbenzimidazole, can solve problems such as complicated operation, achieve simple operation, shorten synthesis steps, and overcome defects and insufficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
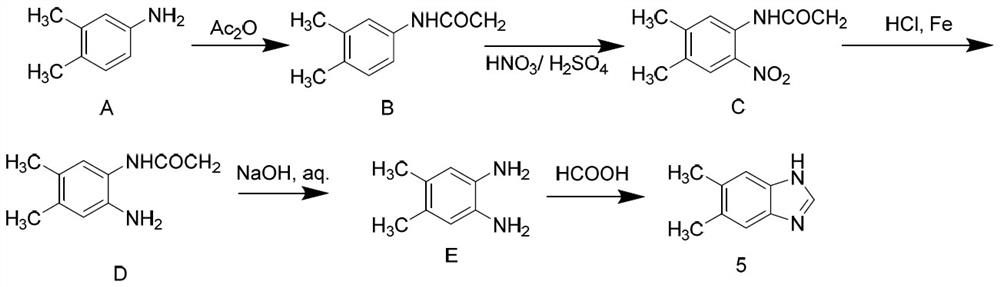
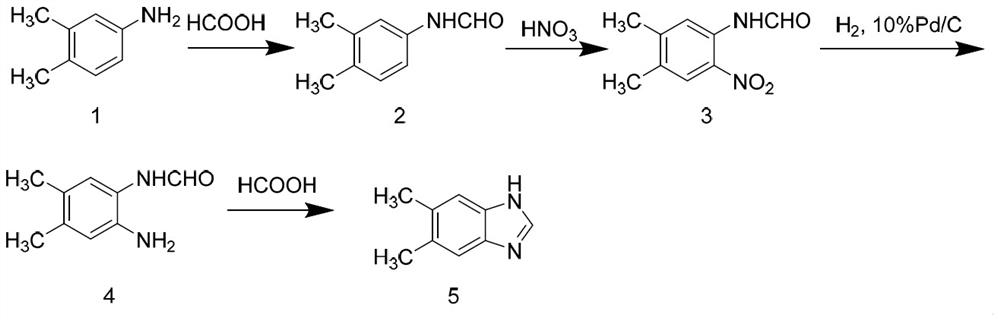
[0017] See attached figure 2 , a preparation method of 5,6-dimethylbenzimidazole, comprising the following steps.
[0018] Step A. Preparation of N-(3,4-dimethylphenyl)formamide: Add 3,4-dimethylaniline into the flask, raise the temperature to 50-90°C, add formic acid dropwise into the flask and react 0.1-3 hours, and then evaporated to dryness under reduced pressure to obtain N-(3,4-dimethylphenyl)formamide.
[0019] Wherein, the ratio of 3,4-dimethylaniline and formic acid added to the flask is 1:1~1:5.
[0020] Step B, preparation of N-(4,5-dimethyl-2-nitrophenyl) formamide: N-(3,4-dimethylphenyl) formamide, reaction solvent and concentrated nitric acid were added to In the flask, react at 0-25°C for 1-10 hours. After the reaction is completed, add ice water to the system dropwise, stir, filter, and dry to obtain N-(4,5-dimethyl-2-nitrophenyl ) Formamide.
[0021] Wherein, the reaction solvent is concentrated sulfuric acid, glacial acetic acid or dichloromethane, and t...
Embodiment 1
[0029] Example 1: Add 3,4-dimethylaniline (121.2g, 1mol) into the flask, raise the temperature to 55°C, after it is completely dissolved, add formic acid (46.1g, 1mol) dropwise, and after 10min, reduce the temperature at 80°C Pressure distillation and evaporation to dryness gave 149.1 g of an oily liquid with a yield of 99.9% and an HPLC purity of 99.6%.
Embodiment 2
[0030] Example 2: Add 3,4-dimethylaniline (121.2g, 1mol) into the flask, raise the temperature to 75°C, after it is completely dissolved, add formic acid (46.1g, 1mol) dropwise, and after 10min, reduce the temperature at 80°C Pressure distillation and evaporation to dryness gave 147.1 g of an oily liquid with a yield of 98.6% and an HPLC purity of 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com