Preparation method of key intermediate of lorlatinib
A technology of total volume and catalytic system is applied in the field of preparation of 5-fluoro-3-methylisobenzofuran-1-one, a key intermediate of lorlatinib, and can solve the problem of long synthesis route, high cost and high yield. lower problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] The preparation method of lorlatinib key intermediate (formula IV)
[0081] The present invention provides a new synthetic route of the intermediate shown in formula IV, through which the synthetic route (or method) greatly shortens the synthetic method provided by the original report, simplifies the operation, improves the yield, and greatly reduced costs. More importantly, the chemical method racemization resolution used in the past was changed, and the biological enzyme was used as the catalyst method, which is environmentally friendly, simple, high yield, and has no side reactions, which greatly improves the product quality.
[0082] Typically, the present invention provides a preparation method of a compound of formula IV, the preparation method comprising the steps of:
[0083] (1) Formula I is subjected to chiral reduction under the catalysis of biological enzymes to obtain chiral formula II
[0084]
[0085] Preferably, the biological enzyme is perakine red...
Embodiment 7
[0135] The preparation of embodiment 7 formula IV compound
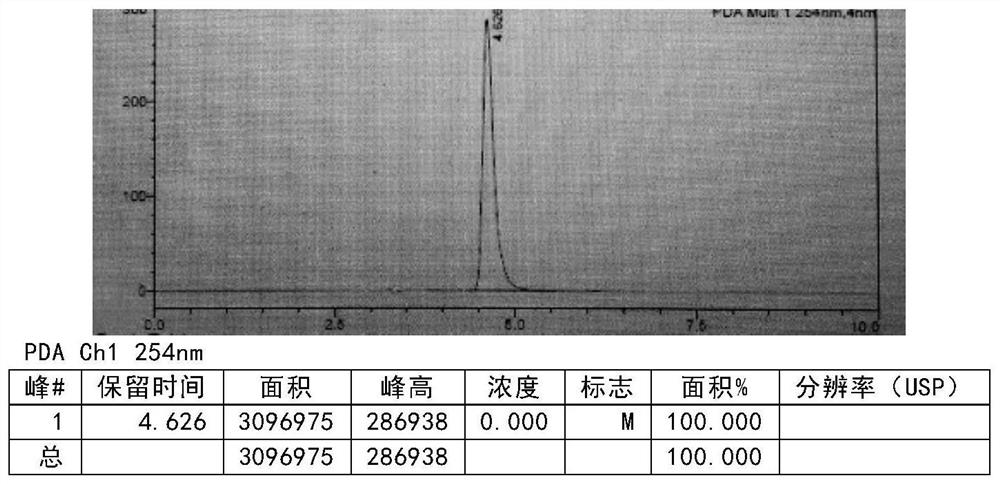
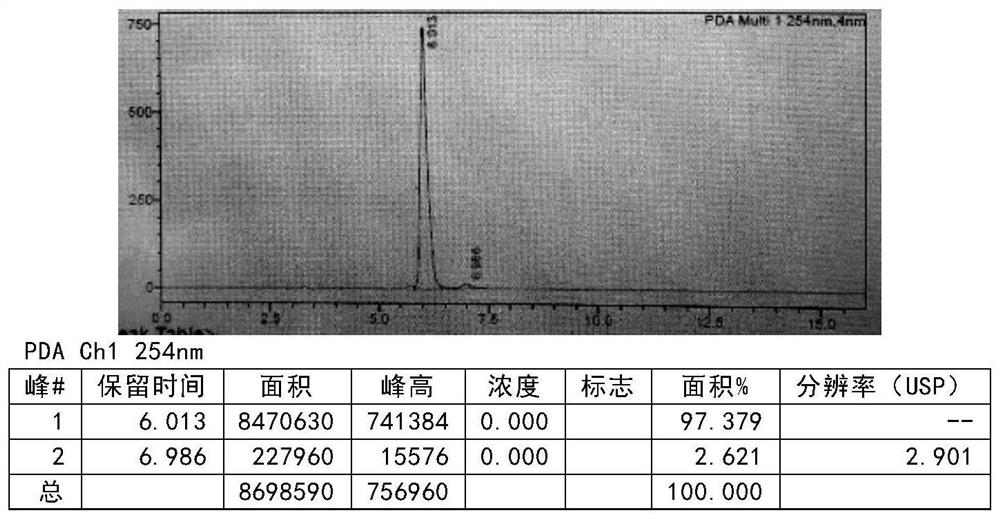
[0136] Add the compound of formula III (50g, 0.27mol, prepared according to the method of Example 4) into the mixed solution of absolute ethanol (450ml) and water (50ml), add concentrated sulfuric acid (264.6g 2.7mol) dropwise, and control the temperature for 20-30 After adding at ℃, react for 2-3h, add the reaction solution dropwise into water (600ml), stir, add dichloromethane (400ml) for extraction, then add dichloromethane (200ml) for extraction and separation, combine dichloromethane, add Pressure distillation gave product (40.14g), yield: 89.1% (relative to formula III) MS (ESI): [M+1] + = 167.17. After testing, the purity is 99.8% (see Figure 4 ).
Embodiment 8
[0137] The preparation of embodiment 8 formula IV compound
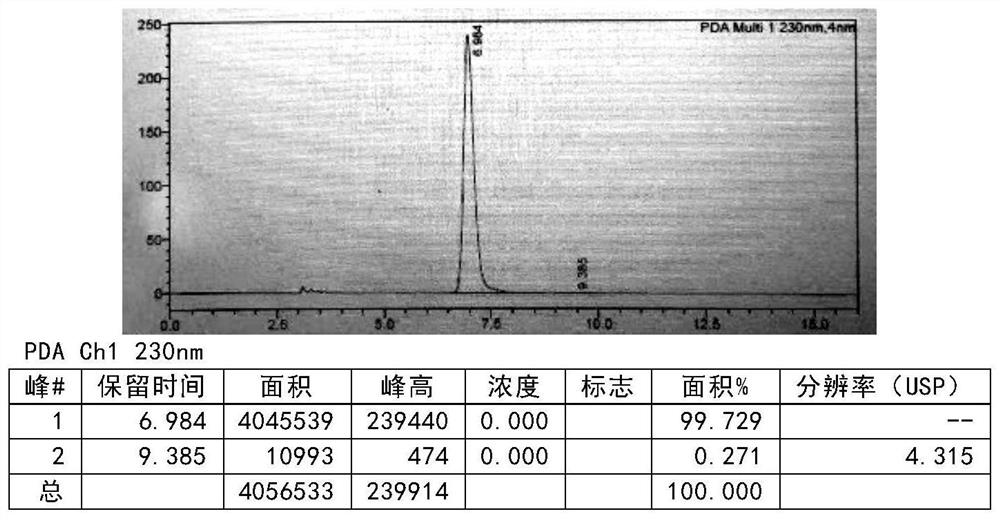
[0138] The compound of formula III (50g, 0.27mol, prepared according to the method of Example 4) was added to the mixed solution of methanol (450ml) and water (50ml), and phosphoric acid (264.6g 2.7mol) was added dropwise, and the temperature was controlled at 20-30°C to complete the addition , reacted for 2-3h, added the reaction solution dropwise into water (600ml), stirred, added dichloromethane (400ml) for extraction, then added dichloromethane (200ml) for extraction and separation, combined dichloromethane, and distilled under pressure to obtain Product (39.3 g), yield: 87.2% (relative to formula III) MS (ESI): [M+1] + = 167.17. After testing, the purity is 98.9% (see Figure 5 ).
[0139] The preparation of comparative example 1 formula III compound
[0140] The compound of formula II (10g, 45.6mmol, obtained by the method of Example 1) was added in ethanol (600ml), added in the autoclave, and palladium ace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com