Liquid preparation containing anti-IL-17 antibody
A liquid preparation, antibody technology, applied in the direction of antibodies, medical preparations containing active ingredients, anti-inflammatory agents, etc., can solve the problems of inconvenient drug administration, poor compliance, dehydration of blood cells and tissue cells, etc. Injection problems, strong patient compliance, and the effect of meeting drug delivery needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 pH Screening (LZM012 20170815-Liq1)
[0074]The pH screening experiment of LZM012 formulation was based on the platform formulation (100mg / mL LZM012, 25mM histidine, 80mg / mL trehalose, 0.5mg / mL polysorbate 80), and multiple pH gradients (5.0, 5.5, 6.0) were designed , 6.5), to screen out the most suitable pH value.
[0075] The prescription design is shown in Table 1.
[0076] Table 1. LZM012 formulation
[0077]
[0078] In the ultra-clean bench, take 12 mL of the platform formulation with pH 5.5, add 0-300 μL of 0.5M HCl or 0.5M NaOH solution to adjust the pH of the platform formulation to 5.0, 5.5, 6.0, 6.5, respectively, and filter with a 0.22 μm syringe filter. Filling volume of 1mL / bottle: Fill the filtered preparation into a 2mL injection bottle, stopper and crimp the cap.
[0079] The DSC test results are shown in Table 2. The results show that: in the range of pH 5.0-6.5, with the increase of pH, Tonset and Tm1 increase; there is basically no ...
Embodiment 2
[0092] Example 2 Viscosity Study (LZM012 20170829-Liq3)
[0093] The study found that the protein hydrophobicity and concentration in the formulation of LZM012 were higher, and the platform formulation (100mg / mL LZM012, 25mM histidine, 80mg / mL trehalose, 0.5mg / mL polysorbate 80, pH 6.0) was found. The viscosity of the liquid is 14cP, which exceeds the internal control standard by 10cP. Consider adding a viscosity reducer to reduce the viscosity of the liquid.
[0094] The purpose of this example is to investigate the viscosity-reducing ability of NaCl and Arginine. The prescription and viscosity test results are shown in Table 4.
[0095] Table 4. LZM012 20170829-Liq3 viscosity test results
[0096]
[0097] Take the required amount of protein stock solution (130mg / mL LZM012, 25mM histidine, 80mg / mL trehalose, 0.5mg / mL polysorbate 80), add preparation buffer 1 (25mM histidine, 80mg / mL seaweed) respectively Sugar, 39.15 mg / mL NaCl, 0.5 mg / mL polysorbate 80), formulation...
Embodiment 3
[0099] Example 3 Prescription Screening (LZM012 20170925-Liq4)
[0100] Referring to biological products listed at home and abroad, the commonly used concentration of polysorbate 80 (PS80) is 0.05mg / mL-2mg / mL. Within this range, 0.5mg / mL PS80 was selected to be added to the LZM012 formulation to achieve solubilization and prevent protein aggregation and adsorption.
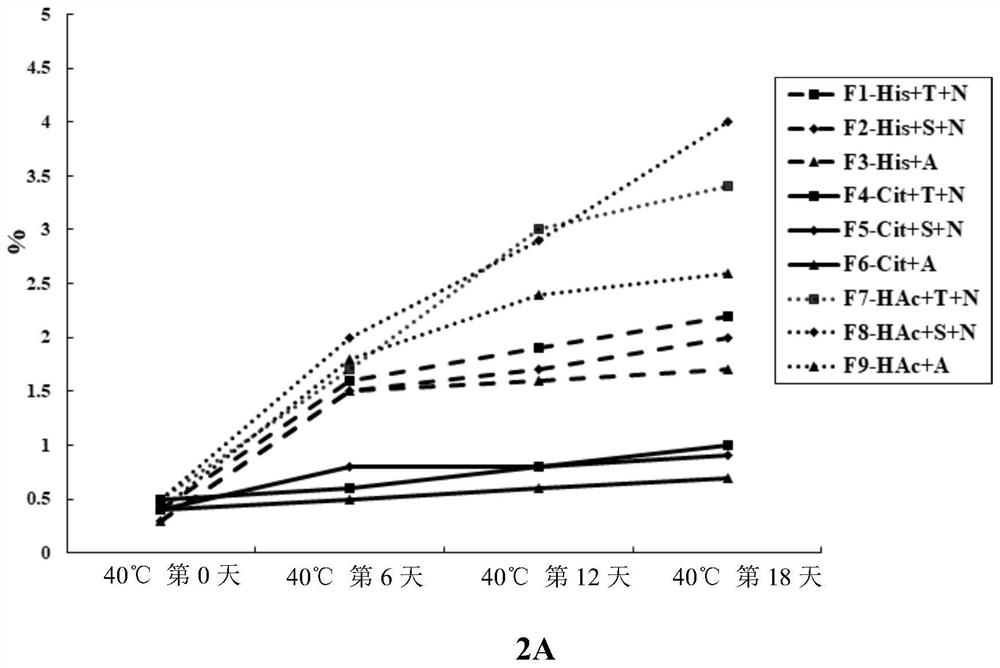
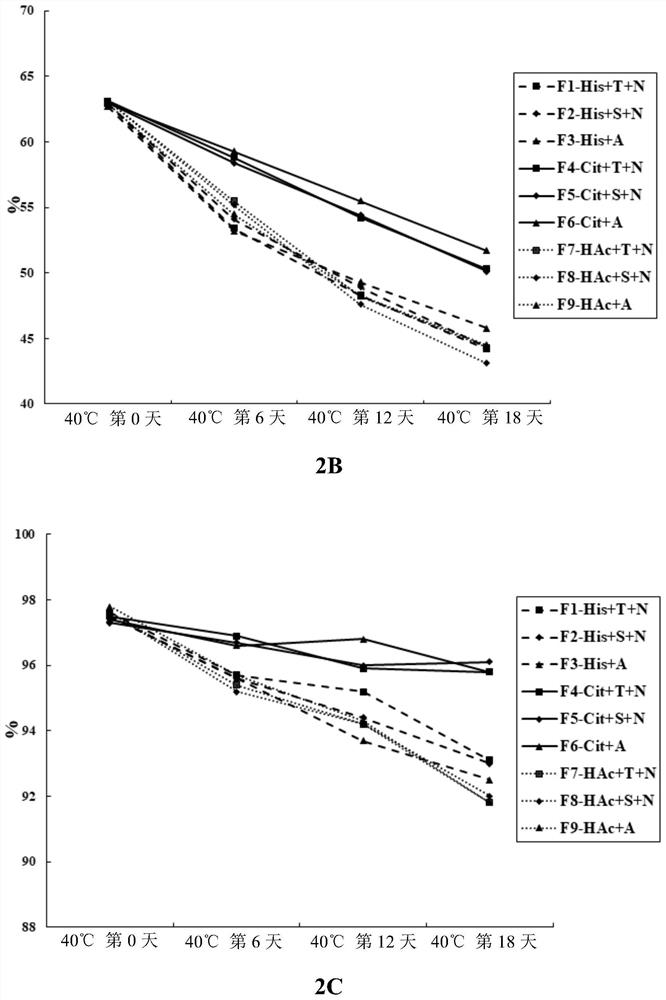
[0101] On the basis of determining pH 6.0 and 0.5mg / mL PS80, continue to screen the types of buffer systems and stabilizers, mainly examining three buffers: Histidine, Citrate, and Acetate (HAc). The system and trehalose (Trehalose), sucrose (Sucrose), arginine (Arginine) three stabilizers. Due to the high viscosity of LZM012, 9mg / mL NaCl was added to reduce the viscosity in the formulations without arginine in this example, and the specific formulation information is shown in Table 5.
[0102] Table 5. LZM012 20170925-Liq4 Prescribing Information
[0103]
[0104] Take an appropriate amount of LZM012 puri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com