Preparation method of norsesquiterpenoids in chimonanthus salicifolius
A technology of sesquiterpenoids and compounds, which is applied in the field of preparation of norsesquiterpenoids in Wintersweet, can solve the problems of small quantity and pharmacological activity research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of wax plum medicinal material and the extraction and separation of norsesquiterpene compounds 1 to 4 include forward and reverse phase column chromatography and high pressure liquid chromatography purification, which specifically adopts the following steps:
[0036] 1. The preparation of the medicinal material of Chimerus willow: dry the branches and leaves of Wintersweet willow, grind them, add 95% ethanol with a volume ratio of 1:3 to extract three times, and obtain the extracts of the branches and leaves after concentration. The extract of branches and leaves is dissolved in hot water, wherein the aqueous solution of extracts of branches and leaves is extracted three times with ethyl acetate, combined and concentrated to obtain ethyl acetate layer extract.
[0037] 2. Column chromatographic separation: the ethyl acetate extract was successively used MCI, and the eluent was methanol-water (50%-100% gradient elution), and a total of 4 parts were obtaine...
Embodiment 2
[0039] Structural identification of compound 1
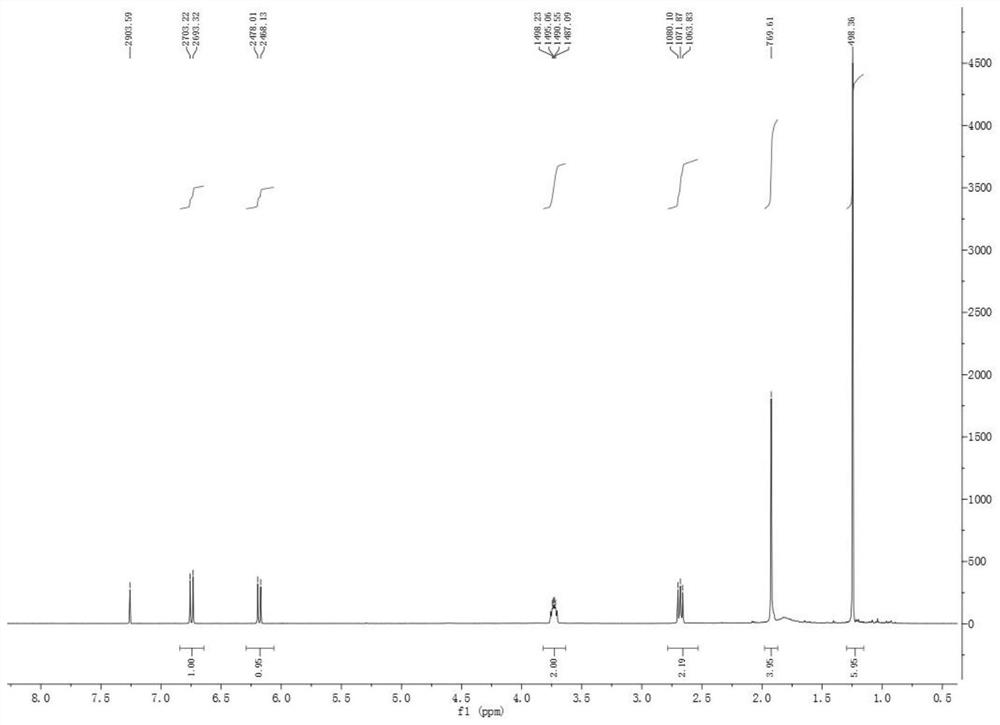
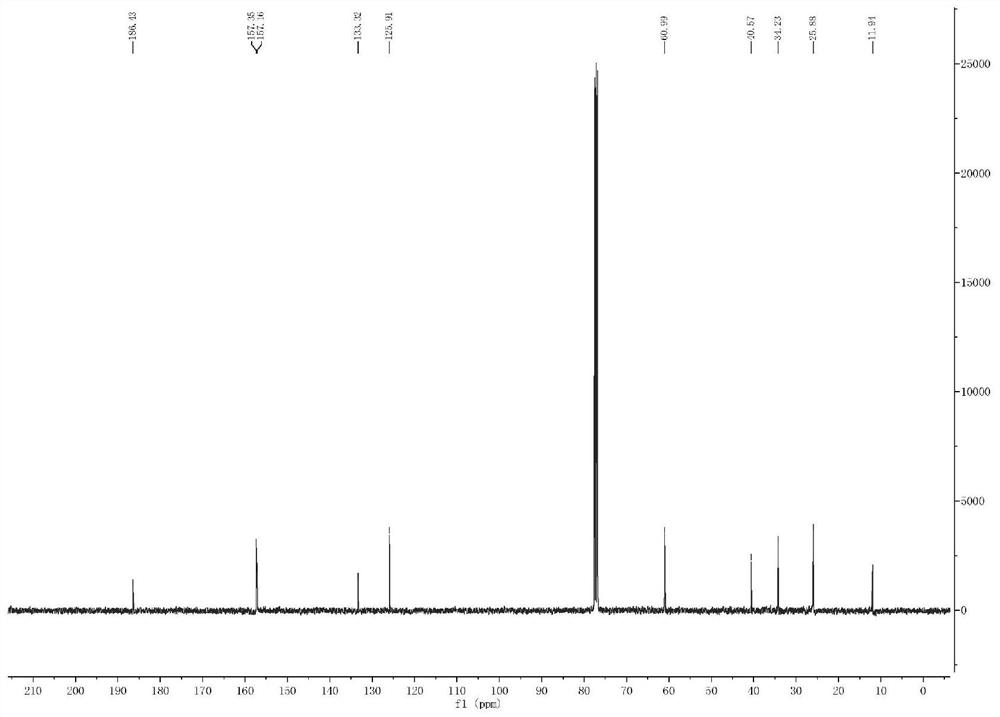
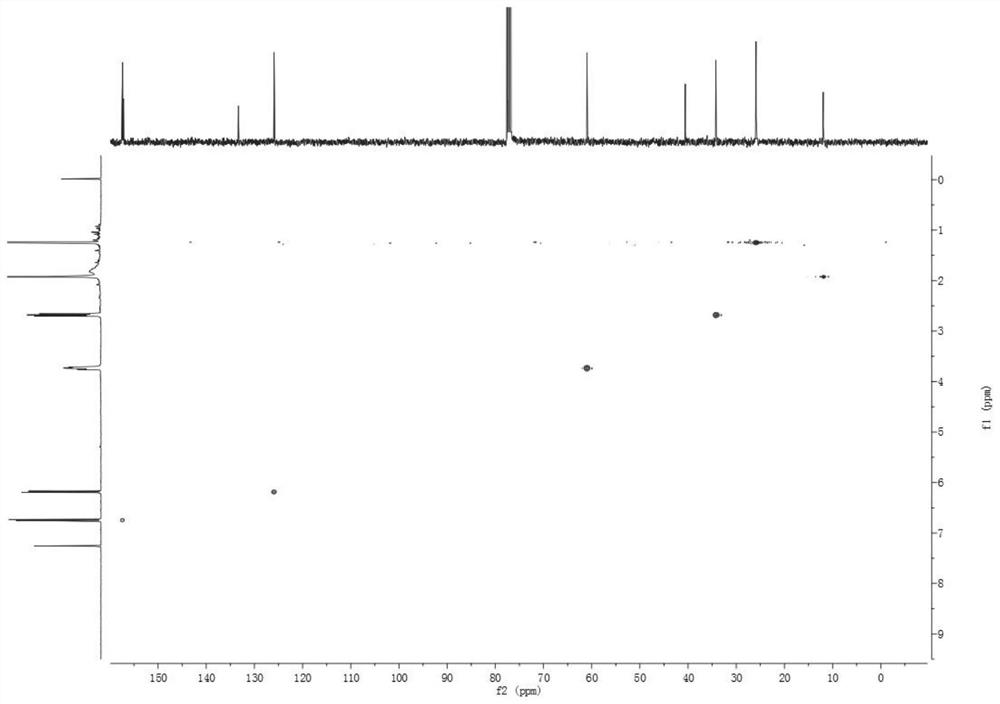
[0040] Compound 1 is a new compound, and its structure was identified by means of modern techniques such as ESIMS and NMR. White amorphous powder. Low-resolution mass spectrometry ESI(+)MS gives molecular ion peak m / z 180.9[M+H] + , combined with NMR data to infer that its molecular formula is C 11 h 16 o 2 , with an unsaturation of 4. IR(KBr)ν max : 3412, 2970, 1656, 1617, 1040, 834cm -1 Indicates the possible presence of conjugated carbonyl and hydroxyl groups. 1 H-NMR (CDCl 3 , 400MHz, Table 1) The spectrum contains 1 pair of cis-alkene hydrogen δ H 6.75 (1H, d, J = 9.9Hz), 6.19 (1H, d, J = 9.9Hz), the methylene hydrogen (δ H 3.74,2H,m), 1 methylene (δ H 2.68, 2H, m), and 3 methyl hydrogens δ 1.25, 1.25, 1.92. 13 C-NMR (CDCl 3 , 100MHz, Table 1) contains 11 C atom signals, including 1 carbonyl (δ C 186.4), 2 double bonds (δ C 157.4, 157.2, 133.3, 125.9), a oxycarbon (δ C 61.0) and five other carbon signals ...
Embodiment 3
[0042] Compounds 2-4 are known compounds, compound 2 is robinlin, compound 3 is (3R, 6R, 7E)-3-hydroxyl-4,7-macrostigmadien-9-one ((3R, 6R, 7E)- 3-hydroxy-4,7-megastigmadien-9-one), and compound 4 is (+)-vomifoliol. Compound 2 is a colorless oil, ESI-MS m / z 199[M+H] + . 1 H-NMR (CDCl 3 , 400MHz) δ: 4.24 (1H, dd, J=14.1, 6.2Hz) 3.72 (1H, m, H-9a), 3.65 (1H, m, H-9b), 2.59 (1H, m, H-8a) , 2.48(1H, m, H-8b), 2.06(1H, dd, J=14.1, 6.2Hz, H-5a), 1.70(1H, t, J=14.1Hz, H-5b), 1.20(3H, s, CH 3 -10), 1.15 (3H, s, CH 3 -11); 13 C-NMR (CDCl 3 , 100MHz) δ: 200.1(C-1), 161.1(C-3), 129.4(C-2), 69.2(C-6), 60.7(C-9), 45.0(C-5), 37.3(C -4), 34.2 (C-8), 29.3 (C-11), 25.3 (C-10), 12.0 (C-7). Compound 3 is a colorless oil, ESI-MS m / z 209[M+H] + . 1 H-NMR (CDCl3 , 400MHz) δ: 6.53 (1H, dd, J=15.8, 10.0Hz, H-7), 6.09 (1H, d, J=15.8Hz, H-8), 5.63 (1H, brs, H-4), 4.27 (1H, brs, H-3), 2.49 (1H, d, J=10.0Hz, H-6), 2.26 (3H, s, CH 3 -10), 1.84 (1H, dd, J=13.4, 5.8Hz, H-2a), 1.62 (3H, s, CH 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com