Preparation method of supramolecular crystal material based on crown ether-Keggin type polyacid
A technology of supramolecular crystals and crown ethers, applied in organic chemistry methods, 6/16 group organic compounds without C-metal bonds, organic chemistry, etc., can solve the problems of difficulty in maintaining structural stability, easy dissociation, and weak force and other problems, to achieve the effect of high yield, simple preparation and stable crystal structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
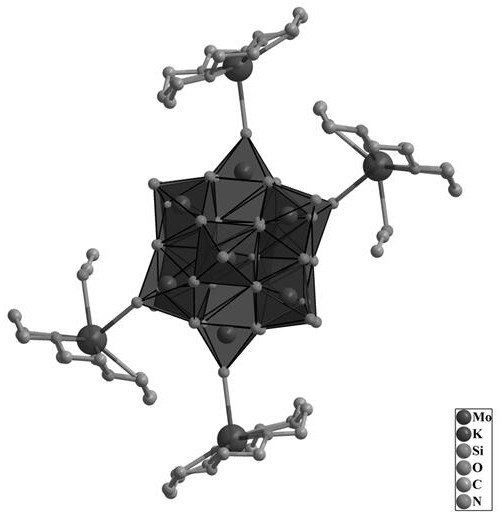
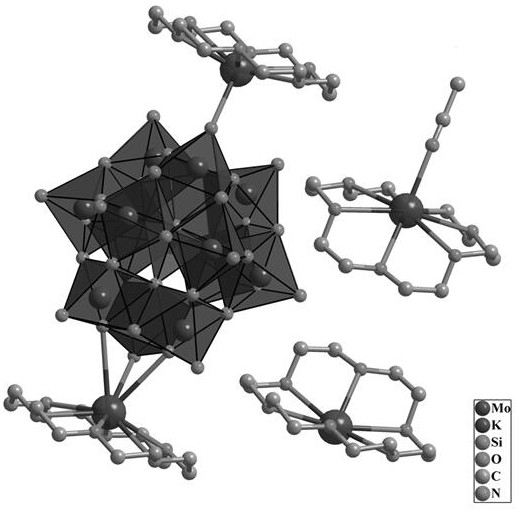
[0039] 455 mg (0.25 mmol) of Keggin-type heteropolyacid H 4 [SiMo 12 o 40 ] dissolved in 125 mL of CH 3 CN, and the temperature of the system was heated to 70°C by a water bath, and the H 4 [SiMo 12 o 40 ] After complete dissolution, 145 mg (1.5 mmol) of KAc and 330 mg (1.25 mmol) of 18-crown-6 were sequentially added. The mixed liquid was stirred for 30 minutes and then filtered while hot. At room temperature, the solution slowly volatilized to obtain yellow-green massive crystals.
[0040] Then after suction filtration, after fully washing with a large amount of deionized water, the crystals were collected in an oven at 60° C., and dried for 24 hours to obtain {[K(H 24 C 12 o 6 )] 2 [K(H 24 C 12 o 6 )(CH 3 CN)] 2 [SiMo12 o 40 ]} 0.559g, referred to as crystal material 1 , yield: 71.7% (based on Mo)
Embodiment 2
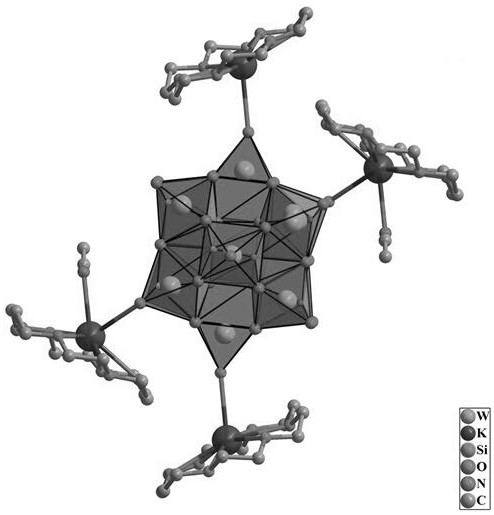
[0042] 720 mg (0.25 mmol) of Keggin-type heteropolyacid H 4 [SiW 12 o 40 ] dissolved in 120 mL of CH 3 CN, and the temperature of the system was heated to 70°C by a water bath, and the H 4 [SiW 12 o 40 ] After complete dissolution, 110 mg (1.5 mmol) of KCl and 330 mg (1.25 mmol) of 18-crown-6 were sequentially added. The mixed liquid was stirred for 30 minutes and then filtered while hot. At room temperature, the solution was slowly volatilized to obtain colorless transparent block crystals.
[0043] Suction filtration, fully washed with a large amount of deionized water and then suction filtered, after fully washed with a large amount of deionized water, the crystals were collected in an oven at 60 ° C, and dried for 24 hours to obtain {[K(H 24 C 12 o 6 )] 2 [K(H 24 C 12 o 6 )(CH 3 CN)] 2 [SiW 12 o 40 ]} 0.845g, referred to as crystal material 2 , Yield: 81% (based on W).
[0044] The crystallographic parameters of the crystal materials prepared in the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com