A kind of gaseous iodine adsorption material with nickel foam as skeleton and its preparation method and application
A foam nickel, iodine adsorption technology, applied in chemical instruments and methods, other chemical processes, nuclear engineering and other directions, can solve the problems of insufficient adsorption capacity, iodine migration risk, serious physical adsorption phenomenon of iodine adsorption materials, etc. The effect of simple capacity and preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of adsorption material with nickel foam as the skeleton and bismuth as the active site:
[0043] (1) Cutting preparation of nickel foam: Cut the purchased nickel foam with a thickness of 0.5mm to 2.5×2cm 2 size, washed with ethanol ultrasonically and dried;
[0044] (2) Configuration of bismuth salt solution: Weigh 0.7275g of bismuth nitrate pentahydrate, dissolve in the mixed solution of ethanol and ethylene glycol (the volume ratio of ethanol to ethylene glycol is 1:1, each is 7.5mL), stir 30min;
[0045] (3) Solvothermal reaction: the nickel foam obtained in step (1) and the bismuth salt solution obtained in step (2) are placed in a 25ml reaction kettle together, and after standing for half an hour, react at 200°C for 12h;
[0046] (4) After the reaction is finished and the reaction is naturally lowered to room temperature, it is taken out with tweezers and washed several times, and dried to obtain the iodine adsorption material with nickel foam as the ...
Embodiment 2
[0049] This embodiment is an iodine adsorption material with nickel foam as the skeleton and silver as the active site. Compared with Example 1, except that the bismuth nitrate pentahydrate in step (2) is replaced by 0.7275g silver nitrate, other steps unchanged, an iodine adsorption material with nickel foam as the skeleton and silver active sites was obtained.
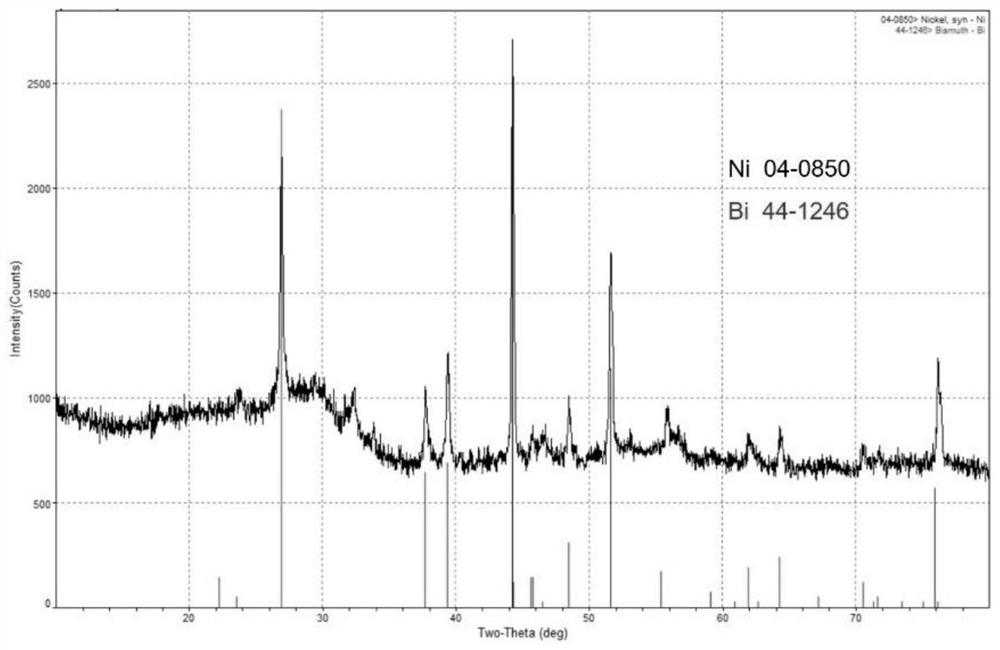
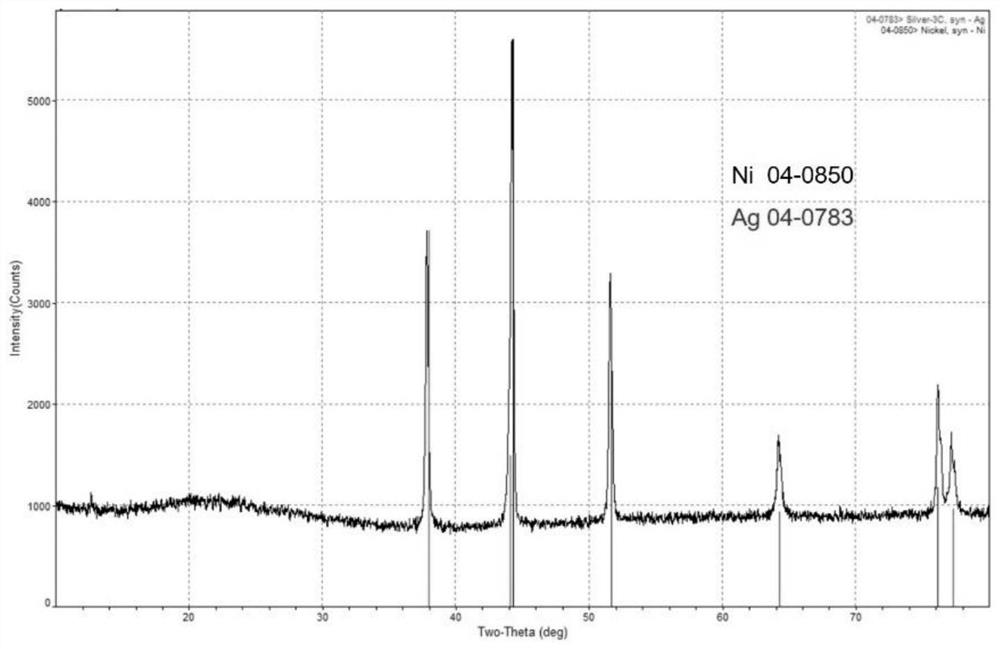
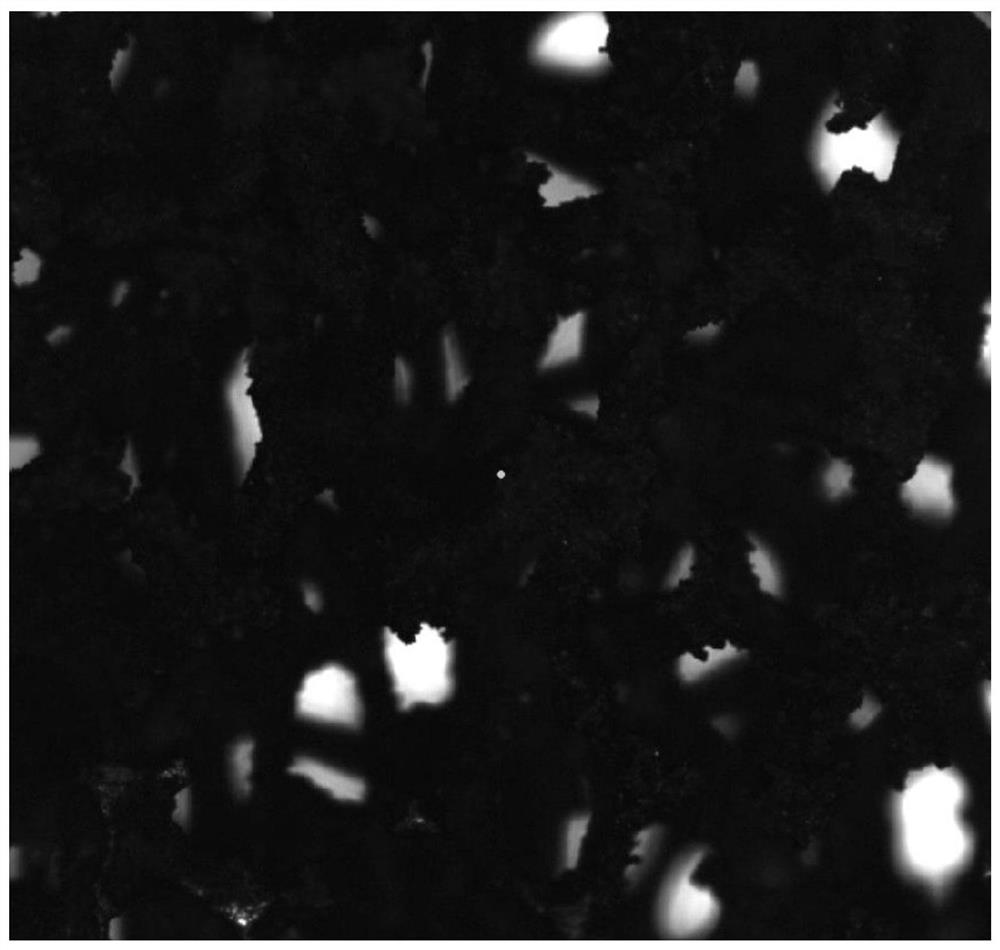
[0050] The adsorption material of embodiment 2 is characterized, and XRD collection of lines is as follows figure 2 As shown, all the diffraction peaks can be attributed to the simple substance of silver (PDF No.04-0783) and the nickel substance of the nickel foam framework (PDF No.04-0850), indicating that under the solvothermal reaction, the silver ions are all reduced to Silver is single substance and successfully loaded onto the nickel framework. Optical microscopy results such as Figure 4 As shown, it can be seen that a layer of silver particles has been firmly wrapped on the surface of the nickel foam, form...
Embodiment 3
[0052] According to the preparation process of Example 1, the solvent in step (2) was replaced with a mixed solution with a volume ratio of ethanol and ethylene glycol of 1:2 and 2:1, respectively, to prepare an adsorption material whose active site was bismuth.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com