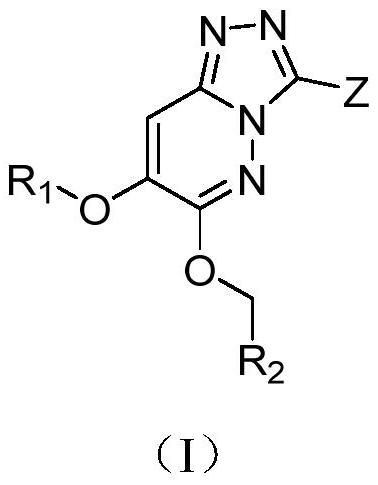
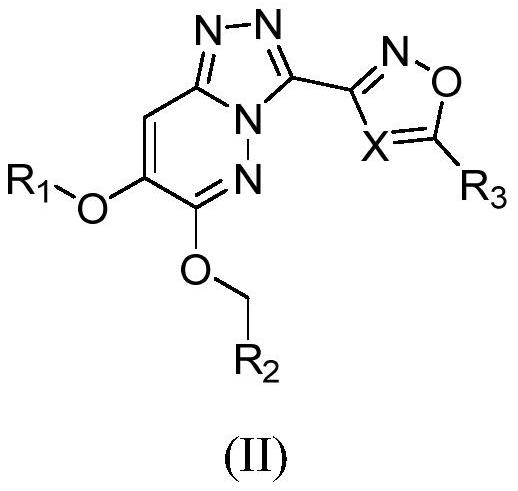
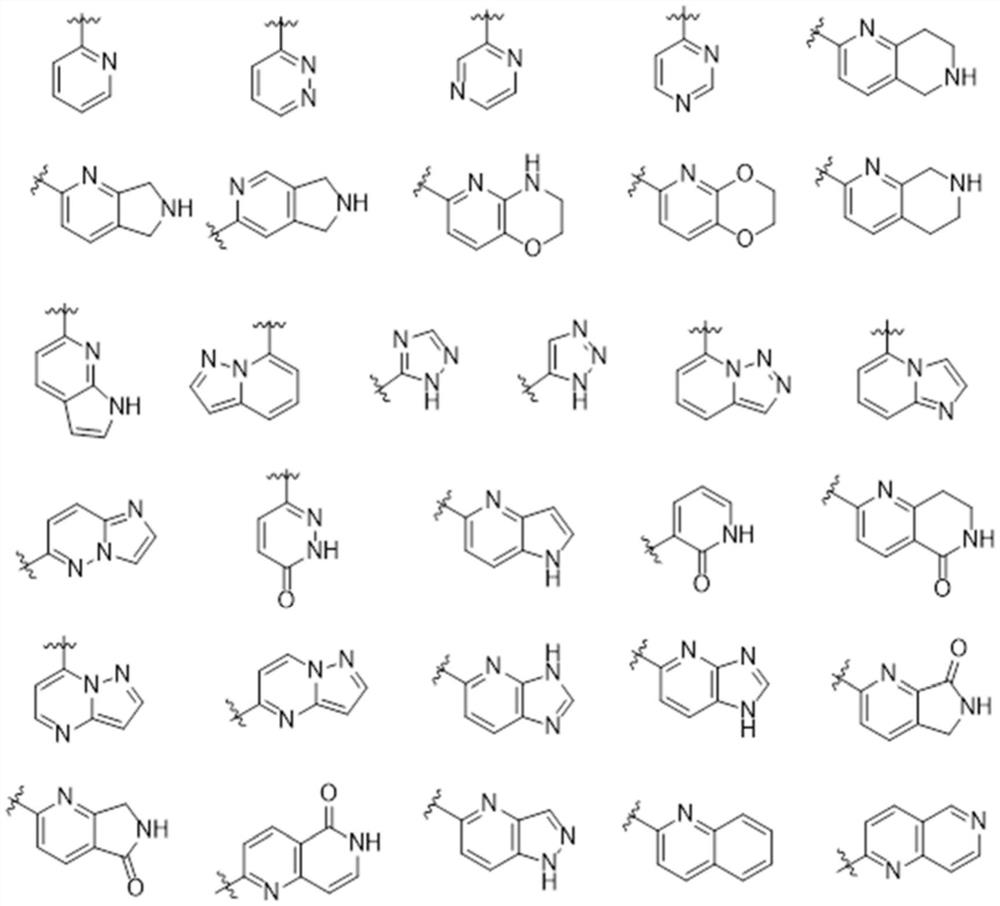
Triazolopyridazine derivative as well as preparation method, pharmaceutical composition and application thereof
A technology of compounds and hydrates, applied in the field of triazolopyridazine derivatives, which can solve problems such as animal fear and anxiety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0235] 2) Compound 3 can be prepared by step c, directly heating the corresponding hydrazide and compound 1 in acid catalysis, such as p-toluenesulfonic acid, etc., in various ethers or alcohol solvents, and there will be different Regioisomers arise and require separation. Alternatively, for substrates with weaker reactivity, compound 2 can be obtained by separating and obtaining compound 2 after reacting hydrazide with compound 1 under similar conditions as in step b, and then heating the ring closure in alcohols, acetic acid and other solvents through step d, Compound 3 was obtained after separation of the isomers.
[0236] 3) Compound 7 can be obtained from the corresponding ester compound 6 by reduction of step i by sodium borohydride, lithium aluminum hydride in solvents such as ethers and alcohols. In addition, for the ester compound 6 with a complex structure, it can be obtained from the corresponding halogenated heteroaryl ester, or the heteroaryl ester substituted b...
Embodiment 1
[1102] Embodiment 1 (method A)
[1103] 3-(7-methoxy-6-((1-methyl-1H-1,2,4-triazol-5-yl)methoxy)-[1,2,4]triazolo[4 ,3- b] pyridazin-3-yl)-5-methylisoxazole
[1104]
[1105] 3-(6-Chloro-7-methoxy-[1,2,4]triazolo[4,3-b]pyridazin-3-yl)-5-methylisoxazole (60mg, 0.23 mmol), (1-methyl-1H-1,2,4-triazol-5-yl)methanol (26mg, 0.23mmol) and cesium carbonate (147mg, 0.45mmol) were added to acetonitrile (3mL) successively, and the reaction mixture Stir at 50°C for 16 hours. The mixture was poured into ice water, and the precipitated solid was collected by filtration and dried to obtain 51.0 mg of the title compound as a white solid, with a yield of 66% 1 H NMR (400MHz, DMSO-d 6 )δ7.98(s,1H),7.77(s,1H),7.09(s,1H),5.66(s,2H), 3.98(s,3H),3.94(s,3H),2.56(s,3H )LC-MS: m / z[M+H] + =343
Embodiment 11
[1107] 6-(((7-methoxy-3-(5-methylisoxazol-3-yl)-[1,2,4]triazolo[4,3-b]pyridazin-6-yl )oxygen base)methyl)-N-(2,2,2-trifluoroethyl)nicotinamide
[1108]
[1109]6-[7-methoxy-3-(5-methyl-isoxazol-3-yl)-[1,2,4]triazolo[4,3-b]pyridazin-6-yl Oxymethyl]-nicotinic acid (200mg, 0.52mmol), HOBT (210mg, 1.56mmol) and EDCI (210mg, 1.56mmol) were added to 5mL of DMF successively, 2,2,2-trifluoroethylamine ( 1410mg, 1.0mmol) and triethylamine (0.5ml) were sequentially added to the mixture, followed by stirring at room temperature overnight. The mixture was diluted with ethyl acetate (10 mL), washed once with brine (20 mL), dried over anhydrous sodium sulfate, and concentrated. The residue was purified by preparative TLC (dichloromethane / methanol = 15 / 1) to obtain 8 mg of the target compound as a white solid with a yield of 3%. The appearance was a white solid. 1 H NMR (400MHz, DMSO-d 6 )δ9.37-9.29 (m, 1H), 9.06 (d, J = 2.0Hz, 1H), 8.29 (dd, J = 2.2, 8.1Hz, 1H), 7.77 (s, 1H), 7.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com