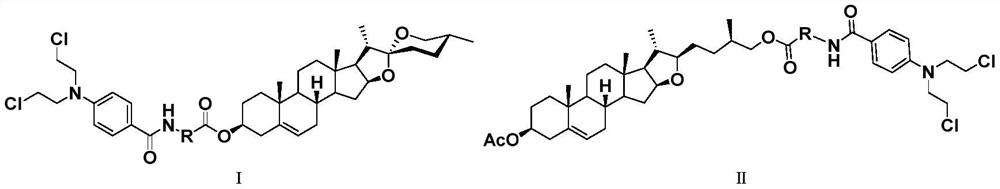
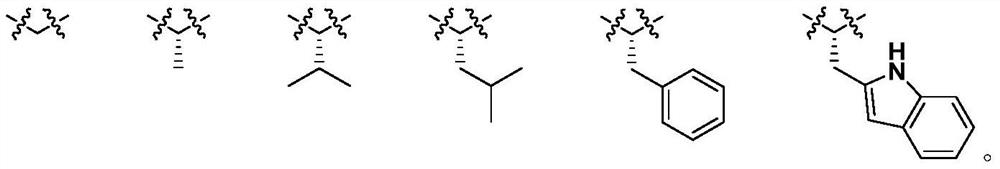
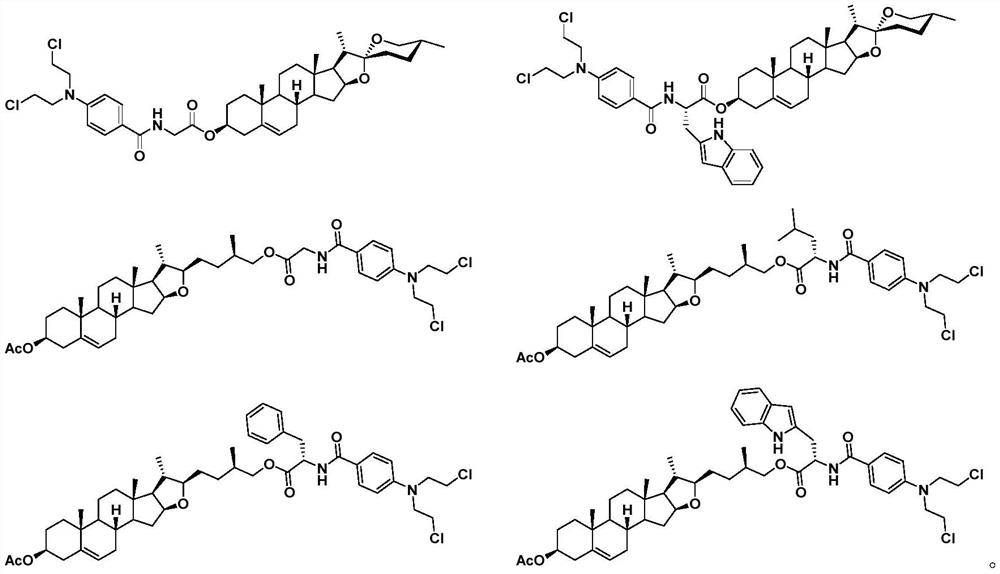
Diosgenin combined nitrogen mustard derivative with anti-tumor activity as well as preparation method and application thereof diosgenin combined nitrogen mustard derivative
A technology of diosgenin and derivatives, which is applied in the field of natural medicine and medicinal chemistry, can solve problems such as limited successful cases, and achieve the effects of high research value, good cytotoxicity, and high clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] Add intermediate 6a (60mg, 0.12mmol) and 5mL CH 2 Cl 2 , after stirring and dissolving, add DMAP (22.0mg, 0.18mmol), EDCI (33.4mg, 0.18mmol) and benzoen mustard 4 (36.5mg, 0.14mmol), stir at room temperature for 8h, thin layer chromatography (petroleum ether: ethyl acetate volume ratio=1.5:1) to monitor the reaction, the basic reaction of raw materials is complete, add 10mL water to the reaction solution, extract three times with 5mL dichloromethane, wash with saturated brine, anhydrous Na 2 SO 4 After drying, filtering, concentrating, and separating and purifying by silica gel column chromatography, the target compound 7a was obtained as a white powder with a yield of 81.2%.
[0051] 1 H NMR (600MHz, CDCl 3 ,δ):7.74(d,J=8.5Hz,2H,Ph-H),6.68(d,J=8.5Hz,2H,Ph-H),5.38(d,J=4.3Hz,1H,H-6 ),4.70(m,1H,H-3),4.41(dd,J=14.9,7.3Hz,1H,H-16),4.19(d,J=4.1Hz,2H,Gly-α-CH 2 ), 3.79(t, J=6.9Hz, 4H, NCH 2 CH 2 Cl,×2),3.65(t,J=6.9Hz,4H,NCH 2 CH 2 Cl,×2),3.47(dd,J=11.0,3...
Embodiment 2
[0055]
[0056] The target compound 7f was prepared according to the synthesis method of Example 1. White powder, yield 79.3%.
[0057] 1 H NMR (600MHz, CDCl 3 ,δ):8.22(br s,1H,Try-NH),7.61(d,J=8.7Hz,2H,Ph-H),7.59(d,J=8.3Hz,1H,Try-Ph),7.35( d,J=8.3Hz,1H,Try-Ph),7.18(t,J=7.7Hz,1H,Try-Ph),7.08(t,J=7.7Hz,1H,Try-Ph),7.03(s, 1H, Try-δ-CH), 6.61 (d, J=8.7Hz, 2H, Ph-H), 5.33 (d, J=4.4Hz, 1H, H-6), 5.08 (dd, J=12.8, 5.6 Hz, 1H, Try-α-CH), 4.59(m, 1H, H-3), 4.41(dd, J=15.0, 7.5Hz, 1H, H-16), 3.76(t, J=7.2Hz, 4H , NCH 2 CH 2 Cl,×2),3.62(t,J=7.2Hz,4H,NCH 2 CH 2 Cl,×2),3.47(dd,J=11.1,3.4Hz,1H,H-26),3.41(d,J=5.3Hz,2H,Try-β-CH 2 ), 3.38(t, J=11.1Hz, 1H, H-26), 1.00(s, 3H, 19-CH 3 ), 0.97 (d, J=7.1Hz, 3H, 21-CH 3 ),0.79(s,3H,18-CH 3 ),0.78(d,J=1.8Hz,3H,27-CH 3 ).
[0058] 13 C NMR (150MHz, CDCl 3 ,δ):171.8(Try-COO),166.5(-CONH),148.8(Ph-C),139.7(C-5),136.2(Try-Ph),129.3(Ph-C,×2),128.0( Try-Ph), 122.9(C-6), 122.7(Try-δ-C), 122.6(Ph-C), 122.3(Ph-C), 119.8(Try-Ph), 119.1(Try...
Embodiment 3
[0061]
[0062] The target compound 12a was prepared according to the synthesis method of Example 1. White powder, yield 84.7%.
[0063] 1 H NMR (600MHz, CDCl 3 ,δ):7.74(d,J=8.8Hz,2H,Ph-H),6.65(d,J=8.8Hz,2H,Ph-H),5.37(d,J=5.0Hz,1H,H-6 ), 4.59 (m, 1H, H-3), 4.30 (m, 1H, H-16), 4.23 (d, J=4.7Hz, 2H, Gly-α-CH 2 ), 4.09(dd, J=10.6, 5.7Hz, 1H, H-26), 3.99(dd, J=10.6, 6.8Hz, 1H, H-26), 3.79(t, J=7.0Hz, 4H, NCH 2 CH 2 Cl,×2),3.65(t,J=7.0Hz,4H,NCH 2 CH 2 Cl,×2),3.30(m,1H,H-22),2.03(s,3H,Ac-CH 3 ),1.03(s,3H,19-CH 3 ),0.99(d,J=6.8Hz,3H,21-CH 3 ), 0.95 (d, J=6.8Hz, 3H, 27-CH 3 ),0.80(s,3H,18-CH 3 ).
[0064] 13 C NMR (150MHz, CDCl 3 ,δ):170.7(Ac-COO),170.6(Gly-COO),166.9(-CONH),148.9(Ph-C),139.8(C-5),129.3(Ph-C,×2),122.5( C-6),111.3(Ph-C,×2),90.2(C-22),83.4(C-16),74.0(C-3),70.4(C-26),65.2(C-17), 57.0 (C-14), 53.5 (NCH 2 CH 2 Cl,×2),50.1(C-9),42.0(Gly-α-CH 2 ), 40.8 (C-12), 40.3 (NCH 2 CH 2 Cl,×2),39.5(C-13),38.2(C-4),38.1(C-20),37.1(C-1),36.8(C-10),32.9(C-24),32.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com