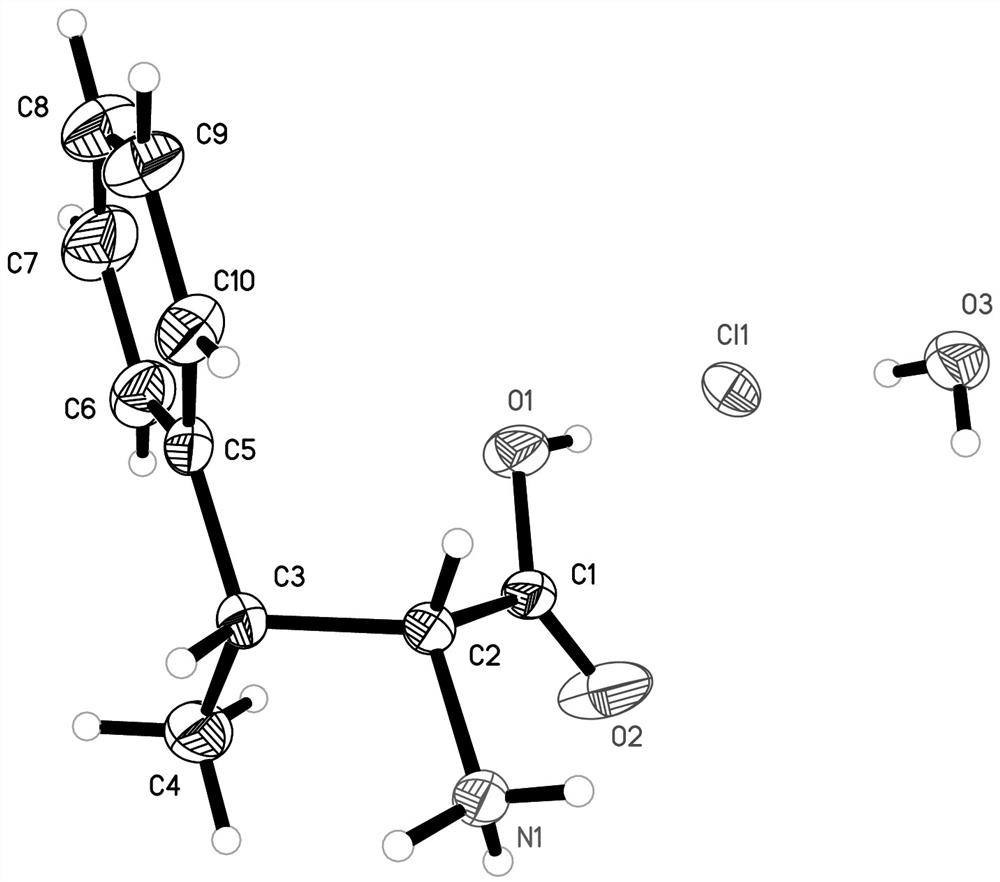
Preparation method of (2R, 3R)-3-methyl-3-phenylalanine
A technology of phenylalanine and diethyl acetamidomalonate, which is applied in the field of preparation of 3-methyl-3-phenylalanine, can solve the difficulty of industrial production and isomer resolution , the selection of expensive reagents and other issues, to achieve quality advantages, cost advantages, and avoid the effects of dangerous reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
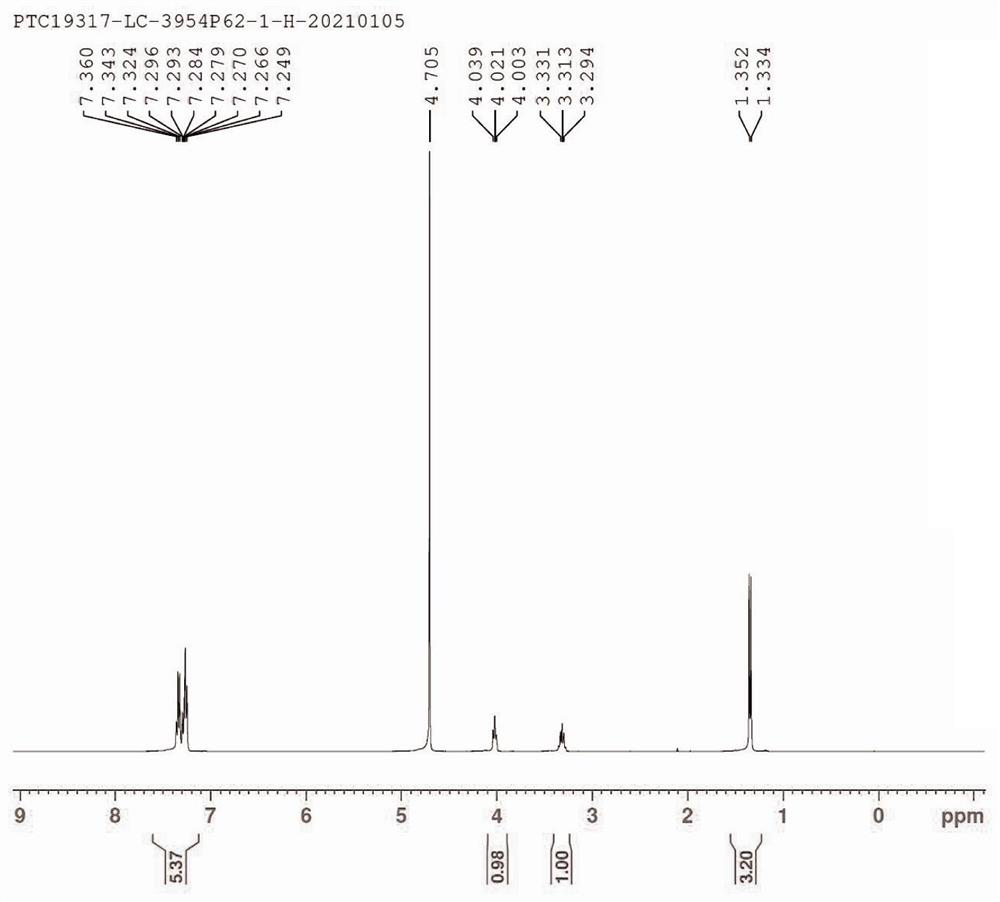
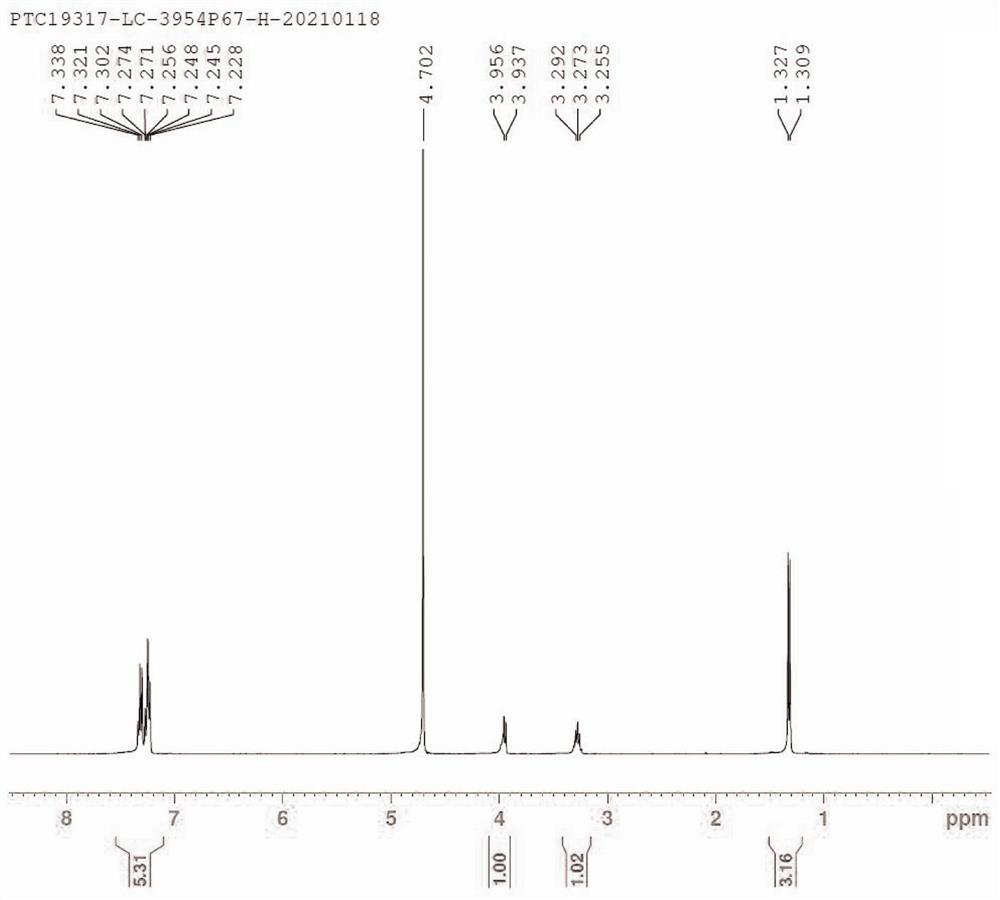
Image
Examples
Embodiment 1
[0043] (1): Add 1085 g (5 mol) diethyl N-acetylaminomalonate and N,N-dimethylformamide (5 L) sequentially into a 10L three-necked flask, stir to dissolve and cool to -10~0 ℃, potassium tert-butoxide (672 g, 6 mol) was added in batches, the temperature was controlled < 0 ℃, and the reaction was stirred for 0.5 hours. Then 1-bromoethylbenzene (925 g, 5 mol) was added dropwise, the temperature was controlled <10°C, and the addition was completed in about 1 hour. The cooling bath was removed, and the reaction was continued to stir at room temperature for 5 hours. The reaction solution was poured into 20L of water, stirred, and a large amount of solids were precipitated. Filter, wash the solid with 10L of water, and dry to obtain intermediate 1 [2-acetamido-2-(1-phenylethyl)diethyl malonate, 1.36 kg, yield 85%].
[0044] (2): Add intermediate 1 [2-acetamido-2-(1-phenylethyl)diethyl malonate, 1.3683 kg, 4.23 mol], 12N concentrated hydrochloric acid (5 L ) and glacial acetic acid (...
Embodiment 2
[0049] (1): Add 108 g (0.5 mol) N-acetylaminomalonate diethyl ester and absolute ethanol (500 mL) in sequence in a 10L three-necked flask, stir to dissolve, then add ethanol solution of sodium ethoxide (20%, w / w, 200 mL, 0.6 mol ), stirred at room temperature for 0.5 hours. Then 1-bromoethylbenzene (92.5 g, 0.5 mol) was added dropwise. After the addition was complete, it was heated to 60°C and stirred for 5 hours. After cooling, the reaction solution was poured into 2L of water, stirred, and a large amount of solids were precipitated. Filter, wash the solid with water, and dry to obtain intermediate 1 [diethyl 2-acetamido-2-(1-phenylethyl)malonate, 105 g, yield 65%].
[0050] All the other steps are the same as in Example 1, and the combined yield of steps (2), (3), (4) and (5) is 20%.
Embodiment 3
[0052] Step (1) is the same as in Example 1; (2) Add intermediate 1 [2-acetamido-2-(1-phenylethyl)diethyl malonate, 100 g, 0.31mol in sequence in a 10L three-necked flask ], 8N concentrated hydrochloric acid (500 L) and 1,4-dioxane (100 mL), stirred and heated to reflux for 12 hours. After cooling, the reaction solution was concentrated to about 150 mL, and crystallized by cooling. Filter, wash the solid with a small amount of ice water, and dry to obtain intermediate 2 (erythro-3-methyl-3-phenylalanine hydrochloride, 21.7 g, yield 32%). All the other steps are the same as in Example 1, and the combined yield of steps (3), (4) and (5) is 25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com