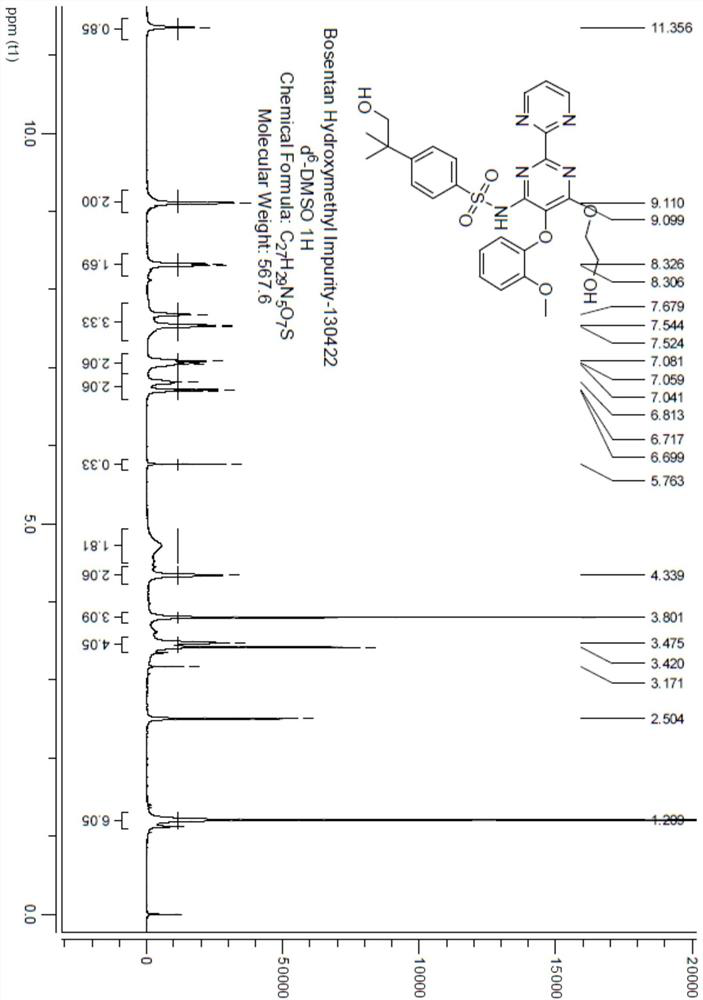
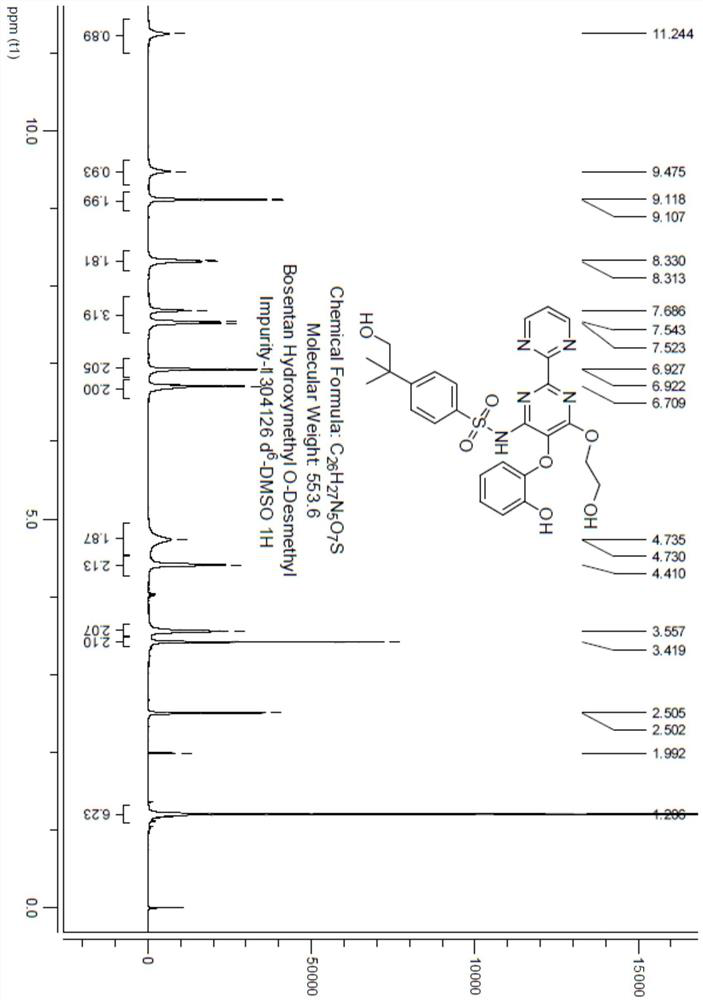
Chemical synthesis method of bosentan metabolite
A technology for chemical synthesis and metabolites, which is applied in the field of chemical synthesis of bosentan metabolites, and can solve problems such as reports on preparation methods of bosentan metabolites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A chemical synthesis method of bosentan metabolites, comprising the following steps:
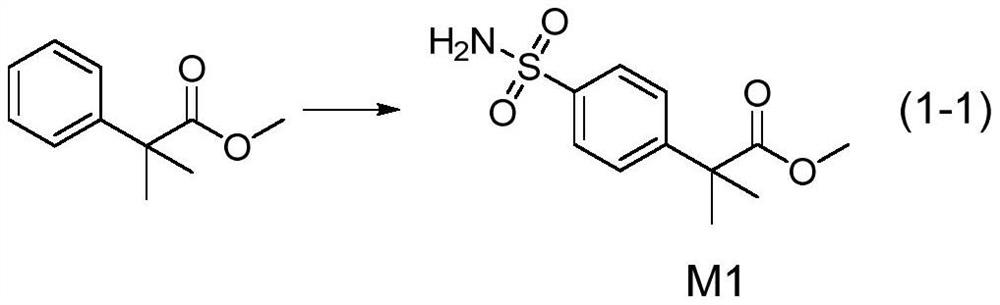
[0028] The first step: the reaction process is shown in formula (1-1)
[0029]
[0030] Add 0.6 g of ammonium chloride to 22 g of chlorosulfonic acid at room temperature, stir for 5 minutes, then slowly drop in 25 g of methyl 2,2-dimethylphenylacetate, raise the temperature to 75°C after the dropping, and keep it warm for 0.5 hours; then Cool down to room temperature and pour into ice water, extract with 200ml ethyl acetate, then wash with saturated brine, mix and stir the washed material with 250ml ice water, then add 50ml ammonia water, stir naturally for 2h, TLC shows that the basic reaction is complete, After standing still, the organic layer was separated, washed with water, dried, evaporated to dryness, and purified by column to obtain 18 g of brown oil M1.
[0031] The second step: the reaction process is shown in formula (1-2)
[0032]
[0033] Dissolve 18g of M1 in 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com