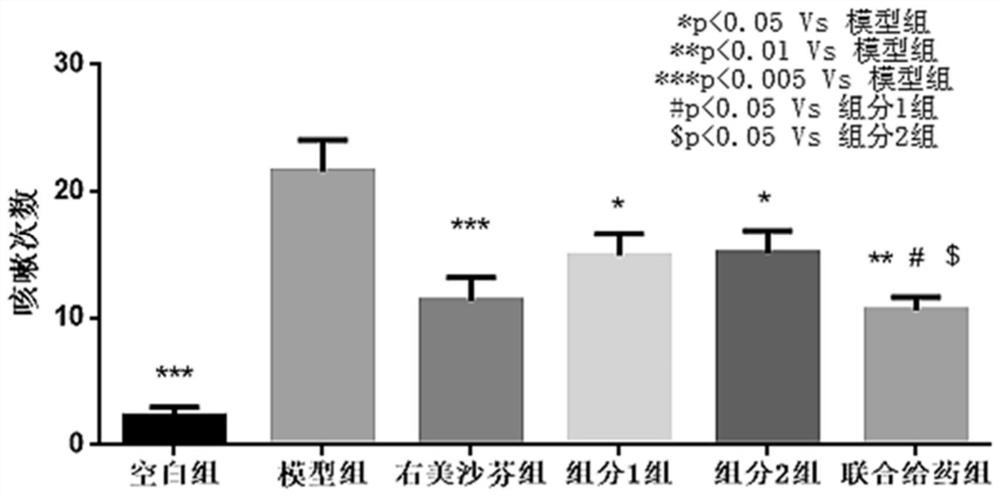
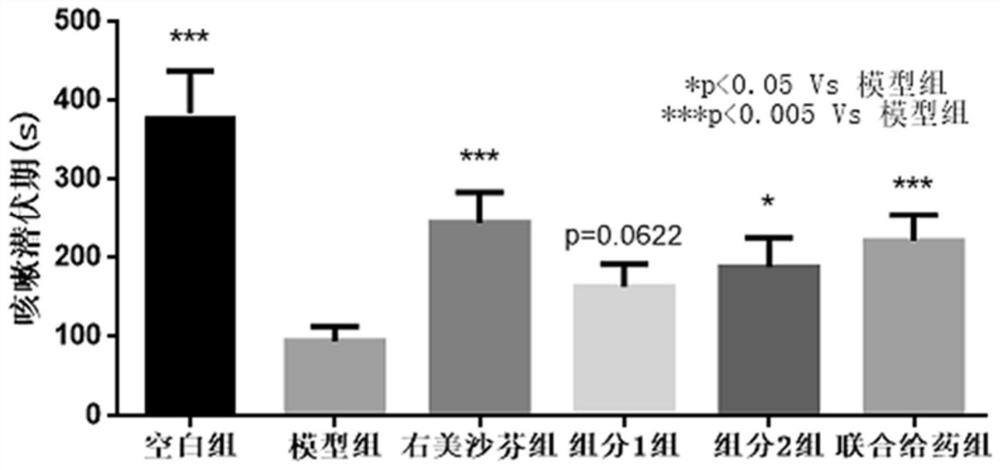
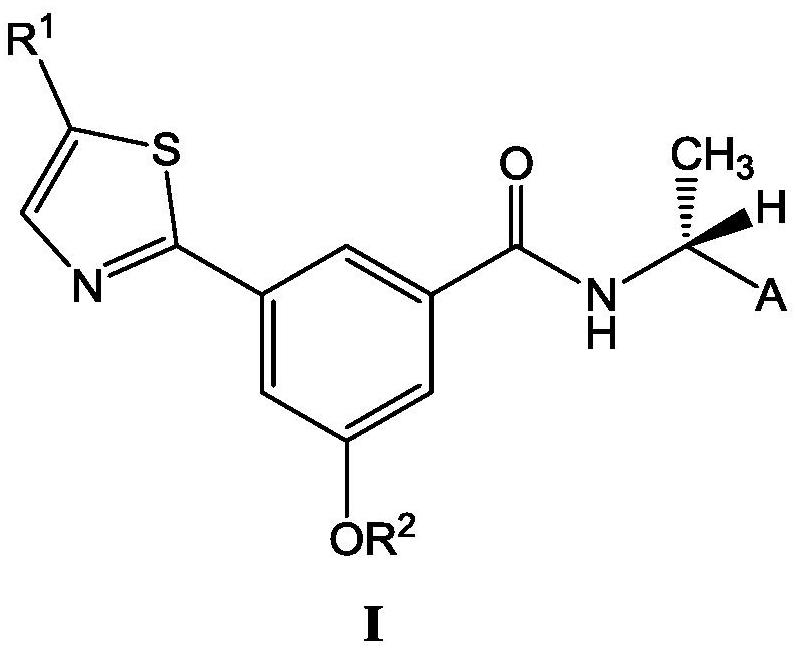
Pharmaceutical composition of P2X3 inhibitor and P2X4 inhibitor and application thereof
A P2X4, inhibitor technology, used in drug combinations, antipyretics, anti-inflammatory agents, etc., to achieve good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0104] Preparation Example 1 Preparation of 3-(5-methyl-1,3-thiazol-2-yl)-5-[(3R)-tetrahydrofuran-3-yloxy]-N-{(1R)-1-[2 -(trifluoromethyl)pyrimidin-5-yl]ethyl}benzamide
[0105] (1) Step 1: Preparation of methyl 3-bromo-5-hydroxybenzoate (intermediate 1A)
[0106]
[0107] A solution of 3-bromo-5-hydroxybenzoic acid (5.0 g, 23.0 mmol) and acetyl chloride (4.0 g, 50.6 mmol) in methanol (50 mL) was stirred at reflux for 16 hours. TLC showed complete conversion of starting material and formation of a new spot. The reaction solution was spin-dried to obtain 4.83 g (91% yield) of the title compound as an off-white solid, which was used in the next step without further purification.
[0108] 1 H-NMR (250MHz, CDCl 3 ): δ[ppm] 7.74(t, J=1.5Hz, 1H), 7.46(dd, J=2.4, 1.3Hz, 1H), 7.25-7.16(m, 1H), 5.57(s, 1H), 3.92( s, 3H).
[0109] (2) Step 2: Preparation of 4-methylbenzenesulfonic acid (3S)-tetrahydrofuran-3-yl ester (intermediate 1B)
[0110]
[0111] A solution of (3S)-te...
preparation example 2
[0138] Preparation Example 2 Preparation of 2-(2-chlorophenyl)-N-{4-[1-(difluoromethyl)-1H-pyrazol-4-yl]-3-sulfamoylphenyl}acetamide
[0139] (1) Step 1: Preparation of 2-bromo-5-nitrobenzenesulfonamide (intermediate 2A)
[0140]
[0141] 2-Bromo-5-nitrobenzenesulfonyl chloride (5 g, 16.6 mmol) was dissolved in 1,4-dioxane (25 ml) and cooled to 0 °C. Aqueous ammonia (100ml, 0.50M, 50mmol) was added slowly and stirring was continued at room temperature until the reaction was complete. The solvent was removed under reduced pressure and dichloromethane was added. The organic phase was washed three times with water. The suspension was filtered (solid was product) and the organic phase was washed with brine. The combined organic phases were dried over sodium sulfate and the solvent was removed under reduced pressure. The crude product was recrystallized from diethyl ether to give 4.4 g of product (94% yield).
[0142] LC-MS (method Y): Rt = 0.45min; MS (ESIpos): m / z = 281 [...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com