Method for catalytically synthesizing 2, 4-dichloronitrobenzene by adopting tetraphenylphosphine iodide
A technology of tetraphenylphosphine iodide and dichloronitrobenzene is applied in the field of preparation of 2,4-dichloronitrobenzene, and can solve the problem of low synthesis rate, low reaction activity, large consumption of ice water, etc. problem, to achieve the effect of improving selectivity and improving reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] HNO by mass ratio 3 :H 2 SO 4 :H 2 O=31:62:7, take 90.5% sulfuric acid and 92.3% nitric acid in a reaction vessel and stir for 2 hours at 22-25°C to prepare a mixed acid.
[0023] Take 600g of m-dichlorobenzene and 200g of nano-tetraphenylphosphine iodide with a particle size of 50nm into the reaction vessel, heat the reaction vessel to 70°C, add the mixed acid dropwise while stirring, and drop the 500ml mixed acid within 1.5 hours. Keep the reaction vessel at 70°C and continue to stir the reaction for 2 hours. After the reaction is completed, let it stand for 2 hours, filter through layers, and recover tetraphenylphosphine iodide nanoparticles. The filtrate is washed with ice water to obtain a white product, and after drying, 554 g of 2,4-Dichloronitrobenzene.
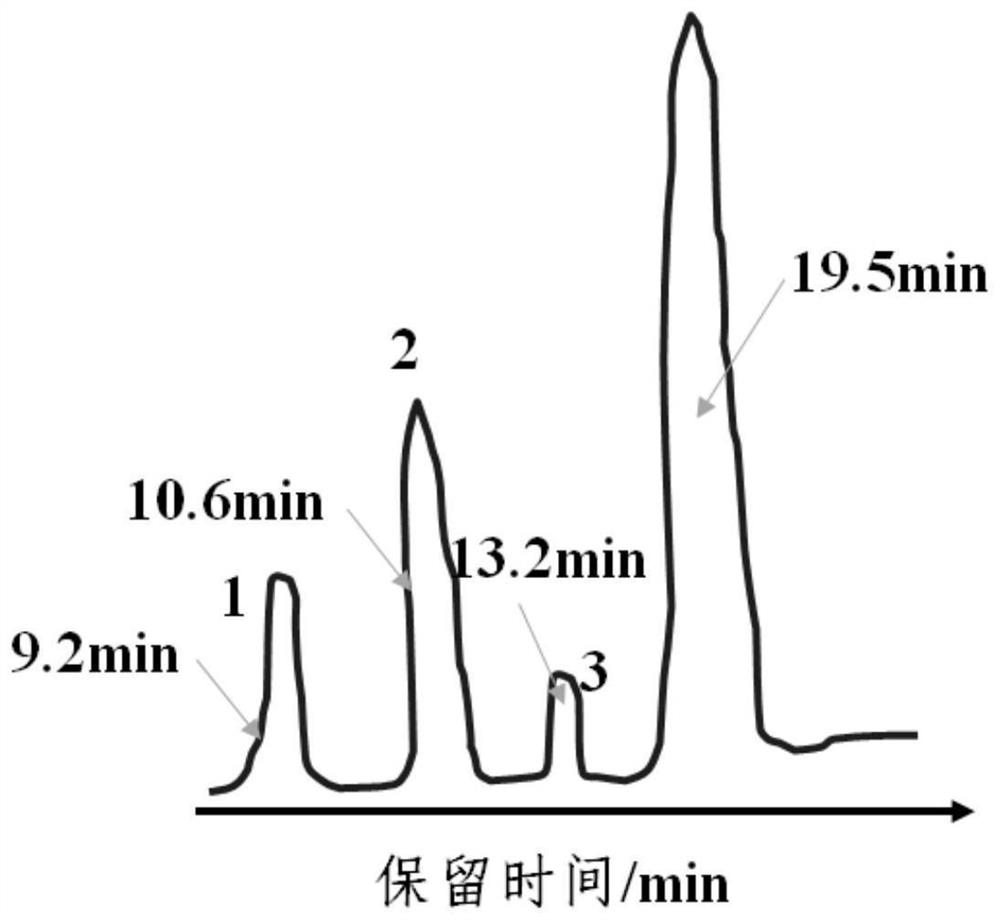
[0024] The prepared 2,4-dichloronitrobenzene was detected by gas chromatography using 102G gas chromatograph and CDMC-1 chromatographic data processor from Shanghai Analytical Instrument Factory. The chrom...
Embodiment 2
[0026] HNO by mass ratio 3 :H 2 SO 4 :H 2 O=31:62:7, take 90.5% sulfuric acid and 92.3% nitric acid in a reaction vessel and stir for 2 hours at 22-25°C to prepare a mixed acid.
[0027] Take 400g of m-dichlorobenzene and 140g of nano-tetraphenylphosphine iodide with a particle size of 20nm into the reaction vessel, heat the reaction vessel to 70°C, add the mixed acid dropwise while stirring, and drop the 300ml mixed acid within 2 hours. Keep the reaction vessel at 70°C and continue to stir the reaction for 2.5 hours. After the reaction is completed, let it stand for 1.5 hours, filter through layers, recover tetraphenylphosphine iodide nanoparticles, wash the filtrate with ice water to obtain a white product, and dry it to obtain 366g of 2,4-dichloronitrobenzene.
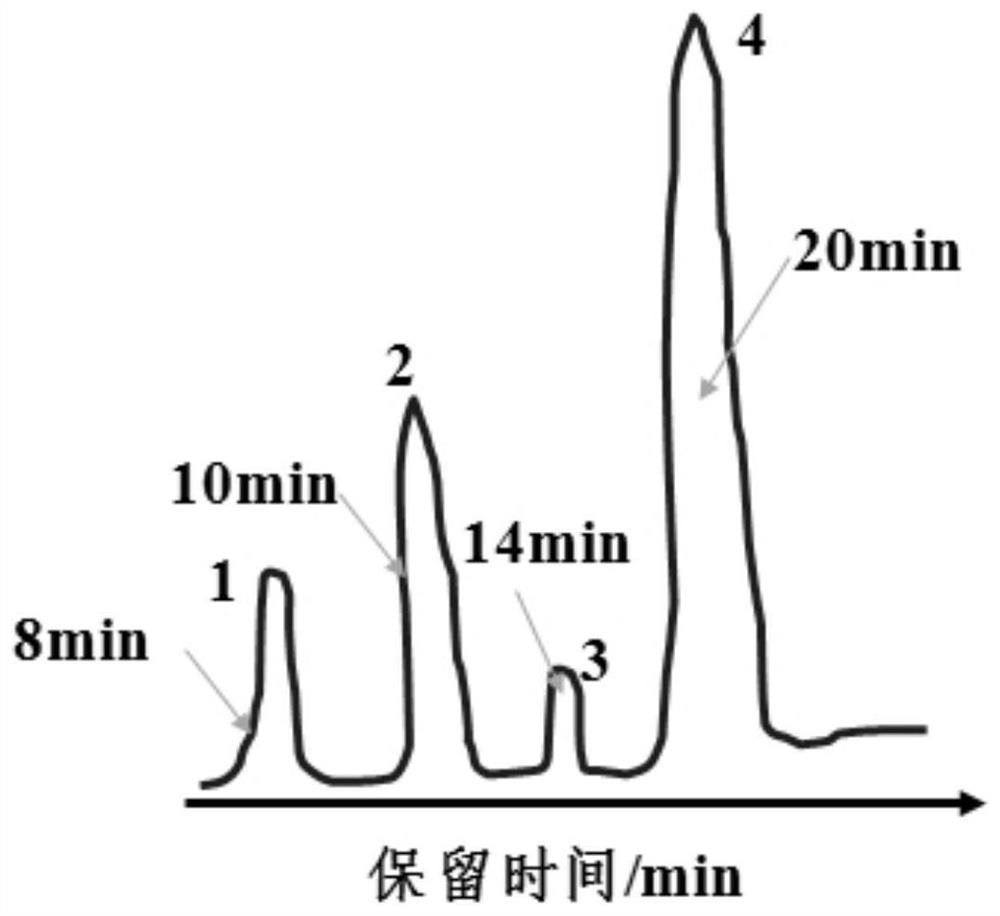
[0028] The prepared 2,4-dichloronitrobenzene was detected by gas chromatography using 102G gas chromatograph and CDMC-1 chromatographic data processor from Shanghai Analytical Instrument Factory. The chromatogr...
Embodiment 3
[0030] HNO by mass ratio 3 :H 2 SO 4 :H 2 O=31:62:7, take 90.5% sulfuric acid and 92.3% nitric acid in a reaction vessel and stir for 2 hours at 22-25°C to prepare a mixed acid.
[0031] Take 800g of m-dichlorobenzene and 280g of nano-tetraphenylphosphine iodide with a particle size of 80nm into the reaction vessel, heat the reaction vessel to 70°C, add the mixed acid dropwise while stirring, and drop the 660ml mixed acid within 2 hours. Keep the reaction vessel at 70°C and continue to stir the reaction for 2.5 hours. After the reaction is completed, let it stand for 1.5 hours, filter through layers, recover tetraphenylphosphine iodide nanoparticles, wash the filtrate with ice water to obtain a white product, and dry it to obtain 741g of 2,4-dichloronitrobenzene.
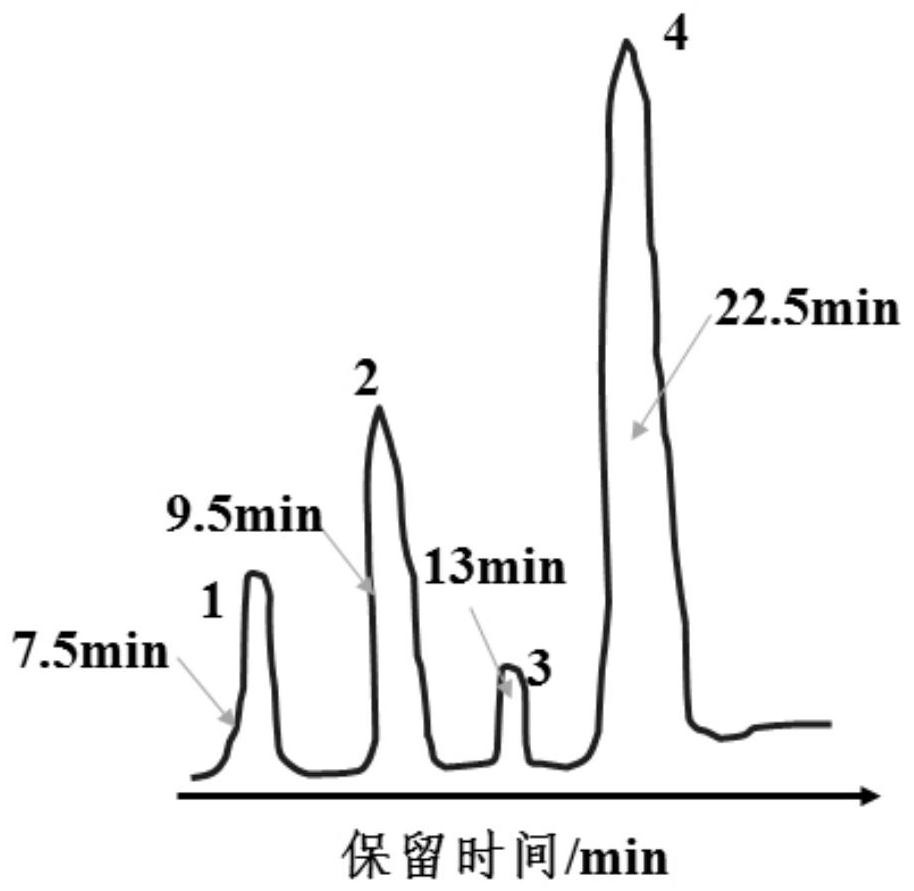
[0032] The prepared 2,4-dichloronitrobenzene was detected by gas chromatography using 102G gas chromatograph and CDMC-1 chromatographic data processor from Shanghai Analytical Instrument Factory. The chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com