Nicotinamide ribose phosphate transferase mutant and application thereof
A technology of phosphoribosyl and nicotinamide, applied in the field of enzyme engineering, can solve the problems of low market competitiveness, high production cost, low enzyme activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This embodiment provides a nicotinamide phosphoribosyltransferase, the preparation method of which is as follows:
[0056] 1. Preparation of recombinant plasmids and recombinant bacteria
[0057] The parent nicotinamide phosphoribosyltransferase used in this example is from Methanobacterium sp.PtaU1.Bin097, and its amino acid sequence is as follows (GenBank: OPY24542.1):
[0058] MNQNLLLMTDSYKASHWLQYPEGTTKIYSYIESRGGKYPETLFFGLQYLLRILEKGINEEDVWEADAFFEVHGVPFNLDGFLYIMNEHDGKLPVEIKAIAEGSVVPAHTPLLTIENTDPSCYWLTCYLETMLLRVWYPTTVATRSWYAKKIIKTYLDQTADDSEAELPSKLHDFGARGASSHESAAIGGMAHLVNFTGSDTVEGVILANKVYKCDMAAFSIPAAEHSTITAWGKENEVEAYRNMLKQFAKPNSLMAVVSDSYDIYNAVENIWGEELRQEVVDSGATIVIRPDSGHPPEIVSKVVKILDEKFGSTENSRGYRVLDNVRVIQGDGVDLDMIHEILDKLKNEGYSASNIAFGMGGYLLQKLNRDTQKFAMKCSYAKVNGKGRDVFKEPVTDKGKTSKRGRINNSLLETVFLDGEIVKEYTLDQVREKAARALE(SEQ IDNo.1)。
[0059] The nucleotide sequence encoding the nicotinamide phosphoribosyltransferase is as follows:
[0060]atgaatcaaaatttgctactgatgactgacagctataa...
Embodiment 2
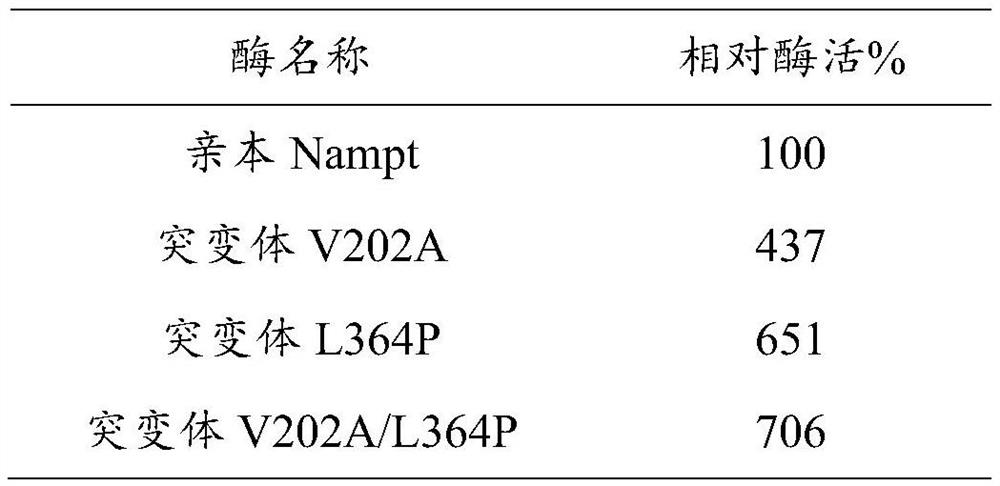
[0079] The final concentration of preparation is 60mM nicotinamide, 25mM PRPP, 18mMMgCl 2 , 15mM KCl, 100mM Tris buffer solution, adjust the pH to 7.5. Take 4 parts of the reaction solution (900 μl each), add 100 μl of parental Nampt with the same protein concentration and supernatant crude enzyme solution of 3 kinds of mutant Nampt, react at 37 ° C for 10 min, then add 100 μl of 25% trichloroacetic acid to terminate the reaction, pass The NMN content in the reaction solution was determined by HPLC, and the specific activity of each enzyme was calculated. Taking the enzyme activity of the parental Nampt as 100% of the relative enzyme activity, the relative enzyme activity of the mutant Nampt was compared, and the results are shown in the following table:
[0080] Table 3. Relative enzyme activity detection results
[0081]
[0082] It can be seen from the above results that the enzyme activity of the single-site mutants provided in the exam...
Embodiment 3
[0084] Preparation of nicotinamide mononucleotide
[0085] Add 90mM nicotinamide, 90mM ribose-5-phosphate, 90mMATP, 20mM MgCl to the reactor 2, 100mM Tris-HCl buffer substrate solution, adjust the pH to 7.0-7.5. Then add catalytic enzyme, the addition amount is respectively: the supernatant crude enzyme liquid of the mutant V202A / L364P of 8ml / L (crude enzyme liquid / substrate solution), the phosphoric acid of 20g / L (lyophilized powder enzyme / substrate solution) Ribose pyrophosphorylase, after stirring evenly, react in a constant temperature water bath shaker. The rotating speed of the shaker was set at 50 rpm, the reaction temperature was controlled at 30°C, and the pH was maintained at 7.0-8.0. After reacting for 4 hours, a solution containing the crude product was obtained, which was filtered, purified, and dried to obtain the final product, which was confirmed to be nicotinamide mononucleotide by hydrogen spectrum and carbon spectrum examination.
[0086] In addition, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com