Influenza vaccine temperature-sensitive gel freeze-dried product for nasal mucosa administration
A technology of thermosensitive gel and influenza vaccine, applied in the field of influenza vaccine thermosensitive gel freeze-dried products, can solve the problems of uncertainty of biological activity, poor stability, influence of thermosensitive properties of thermosensitive gel, etc., and achieve the effect of ensuring drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 Preparation of Influenza Vaccine Thermosensitive Gel Freeze-dried Product and Solvent
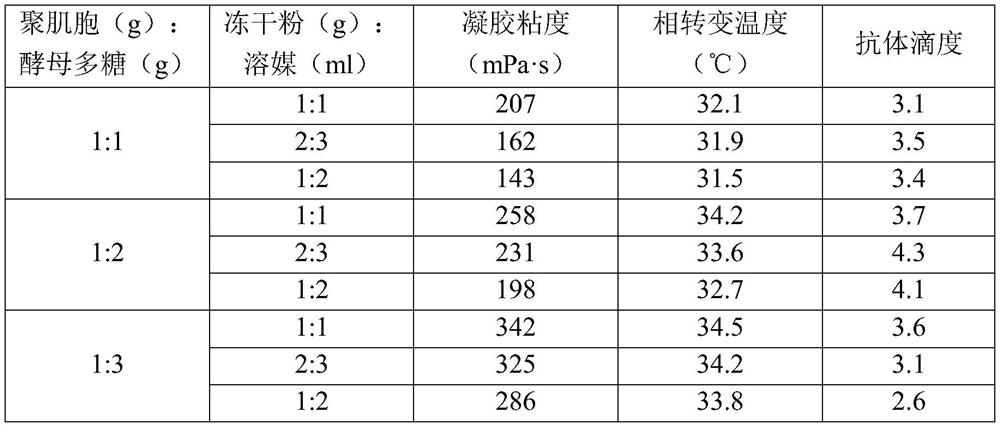
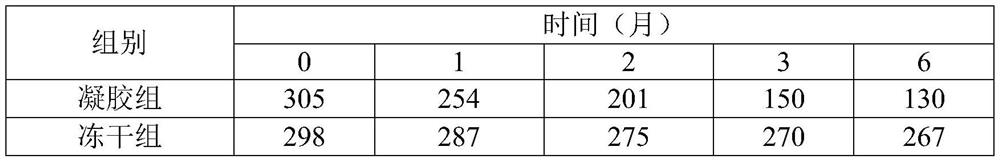
[0021] The influenza vaccine temperature-sensitive gel freeze-dried product to be prepared in the present invention is composed of influenza vaccine and temperature-sensitive gel solids, and the component contents in the temperature-sensitive gel solids are as shown in Table 1:
[0022] Table 1. Solid Content of Influenza Vaccine Thermosensitive Gel
[0023] Gel material (wt%) Gel additive (wt%) Lyoprotectant (wt%) Phosphate buffer matrix (wt%) 80 2.6 to be determined to be determined
[0024] The solvent to be prepared in the present invention consists of vaccine adjuvant and solvent.
[0025] Preparation of influenza vaccine thermosensitive gel: Dissolve the prescribed amount of CS and lyoprotectant in an appropriate amount of PBS, slowly add the prescribed amount of P407 and P188 under magnetic stirring at 4°C, swell at 4°C for 12h-48h, ta...
Embodiment 2
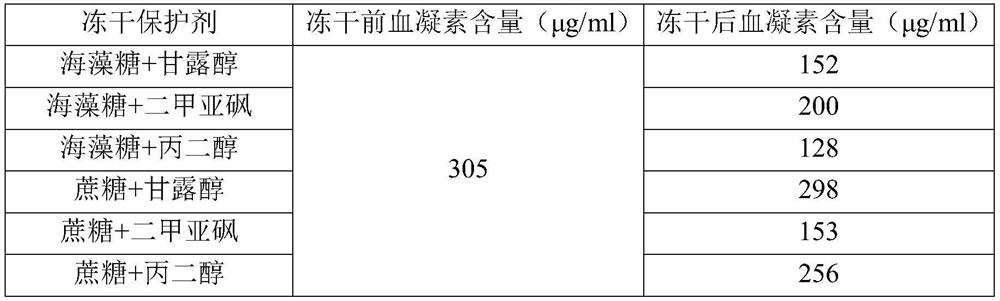
[0028] The impact of the kind of embodiment 2 lyoprotectant on the temperature-sensitive gel of influenza vaccine
[0029] Set the mass proportion of lyoprotectant in the preparation to a certain value, and only change the type of lyoprotectant to explore the influence of different types of lyoprotectant on the performance of influenza vaccine thermosensitive gel.
[0030] By measuring the hemagglutinin content before and after lyophilization, the lyoprotectant is screened, and the screening method is as follows: the standard antigen product is subjected to original times (4 / 4), 3 / 4, 2 / 4, and 0.9% sterile sodium chloride solution. For 1 / 4-fold dilution, add one well for each dilution, add one well for each dilution of vaccine samples, 12 μl per well, place in a horizontal wet box, and diffuse at room temperature for at least 18 hours. After adding the sample, soak the gel plate in 0.9% sterile sodium chloride solution for 60 minutes, take out the gel, place it on the filter pa...
Embodiment 3
[0037] Example 3 The Effect of the Content of Lyoprotectant in the Gel Solids on Influenza Vaccine Thermosensitive Gel
[0038] On the basis of Example 2, only the amount of lyoprotectant was changed to explore the effect of different amounts of lyoprotectant on the performance of the influenza vaccine thermosensitive gel.
[0039] Use a viscometer to measure the gel viscosity of the temperature-sensitive gel, and the test method is as follows:
[0040] Place the prepared gel solution in a beaker with a diameter of not less than 70mm, and place it in a constant temperature water bath at 25°C for 0.5h. Use the NDJ-1 viscometer to measure the viscosity of the sample. When measuring, the reading indicated by the pointer on the scale dial multiplied by the specific coefficient on the coefficient table is the absolute viscosity of the solution. Each group was measured 3 times in parallel, and the results were averaged.
[0041] When the viscosity is between 200 and 300mPa·s, it i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com