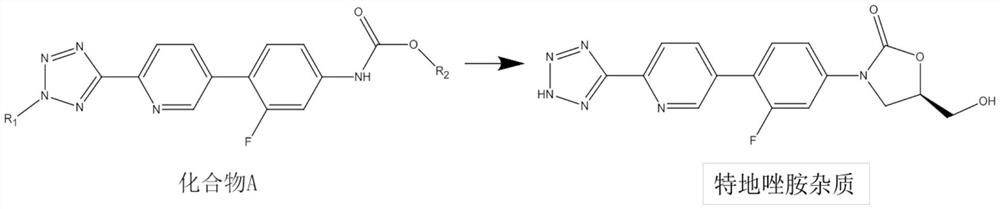
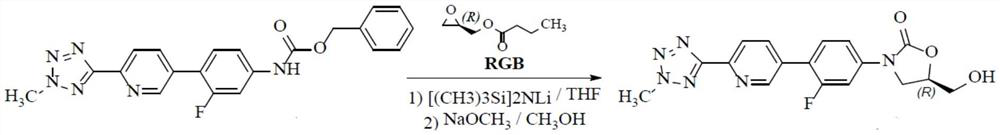
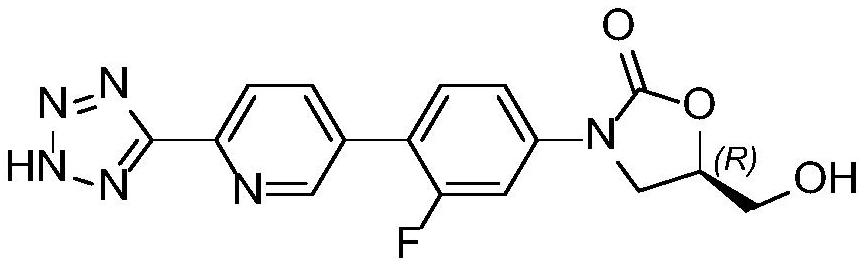
A kind of preparation method of tedizolid impurity
A technology of tedizolid and impurities, which is applied in the field of preparation of tedizolid impurities, and achieves the effect of reasonable synthesis route, few steps and simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation and purification of tedizolid impurities 1
[0030] Synthesis steps: add 100 mL of THF and 5.50 g (13.6 mmol) of compound A (R 1 =-CH 3 , R 2 =-CH 2 Ph), stir and mix well, add 12.8 mL of a THF solution of 1.6 mol / L lithium diisopropylamide at a temperature of 25 to 35 °C, keep at 20 to 30 °C and stir for 1 hour. Add 1.74g (13.6mmol) DMPU under temperature control at 20~30°C, cool down to 0~10°C, then add 1.96g (13.6mmol) (R)-glycidyl butyrate, first stir at 0~10°C for 30 minutes, then The temperature was raised to 20-30° C. and the reaction was stirred, and the reaction was monitored by TLC until the end of the reaction. Through HPLC analysis, 3.01 g of tedizolid impurity was obtained, and the yield was 62%.
[0031] Preparation steps of crude tedizolid impurity: after the reaction is completed, add 0.6 mL of saturated methanol solution of sodium methoxide, stir at 20-30 °C for 1 hour, then cool down to 0-10 °C, add 15 mL of saturated aqueous...
Embodiment 2
[0034] Example 2: Preparation and purification of tedizolid impurities 2
[0035] Synthesis steps: add 100 mL of 2-methyltetrahydrofuran and 5.45 g (13.6 mmol) of compound A (R 1 =-OCH 2 CH 2 CH 3 , R 2 =-CH 2 CH 2 CH 3 ), stir and mix well, add 17 mL of a 2-methyltetrahydrofuran solution of 1.6 mol / L n-butyllithium at a temperature of 25 to 35 °C, keep at 20 to 30 °C and stir for 1 hour. The temperature was controlled at 20~30°C, 17.4g (136mmol) DMPU was added, the temperature was lowered to 0~10°C, then 1.39g (13.6mmol) (R)-glycidyl formate was added, and the temperature was stirred at 0~10°C for 30 minutes, and then the temperature was increased. The reaction was stirred at 20-30° C., and the reaction was monitored by TLC until the end of the reaction. Through HPLC analysis, the impurity yield of tedizolid was 60%.
[0036] Preparation steps of crude tedizolid impurity: after the reaction is completed, add 0.6 mL of saturated methanol solution of sodium methoxide, s...
Embodiment 3
[0038] Example 3: Preparation and purification of tedizolid impurities 3
[0039] Synthesis steps: add 100 mL of ether, 6.29 g (13.6 mmol) of compound A (R 1 =-CH 2 Ph, ), stir and mix well, add 15 mL of a THF solution of 1.6 mol / L lithium bis(trimethylsilyl)amide at a temperature of 25 to 35 °C, keep at 20 to 30 °C and stir for 1 hour. Add 8.7g (68mmol) DMPU under temperature control at 20~30°C, cool down to 0~10°C, then add 1.96g (13.6mmol) (R)-glycidyl butyrate, stir at 0~10°C for 30 minutes, then heat up The reaction was stirred at 20-30° C., and the reaction was monitored by TLC until the end of the reaction. Through HPLC analysis, the impurity yield of tedizolid was 60%.
[0040] Preparation steps of crude tedizolid impurity: after the reaction is completed, add 0.6 mL of saturated methanol solution of sodium methoxide, stir at 20-30 °C for 1 hour, then cool down to 0-10 °C, add 15 mL of saturated aqueous ammonium chloride solution to quench the reaction, and control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com