Application of peimine in preparation of medicine for preventing and/or treating ulcerative colitis
A technology for ulcerative colitis and fritillin A, which is applied in the field of medicine, can solve the problems of anti-ulcerative colitis and other problems that no fritillin A has ever seen, and achieve excellent anti-ulcerative colitis activity, prevention and intestinal tissue pathology. Injury, the effect of reducing the rate of weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 Peiminin A Safety Experiment
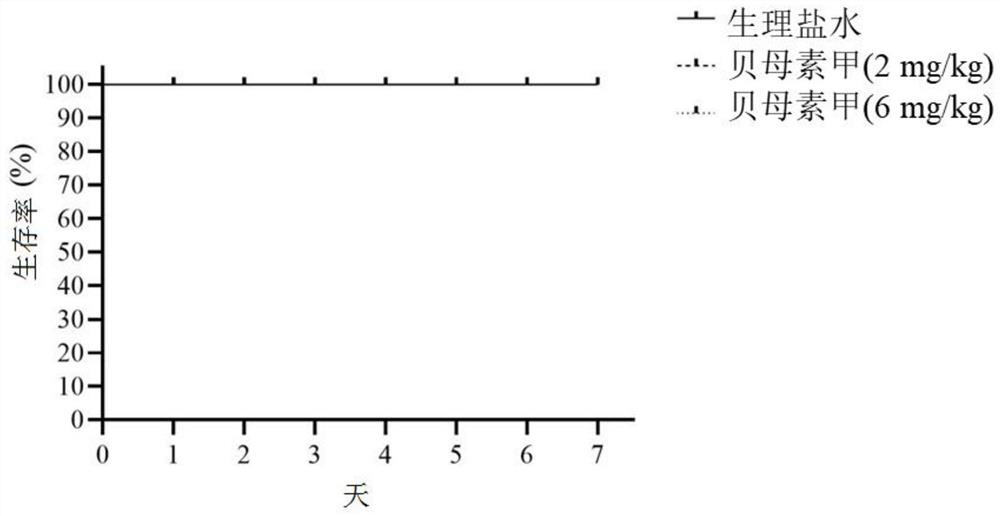
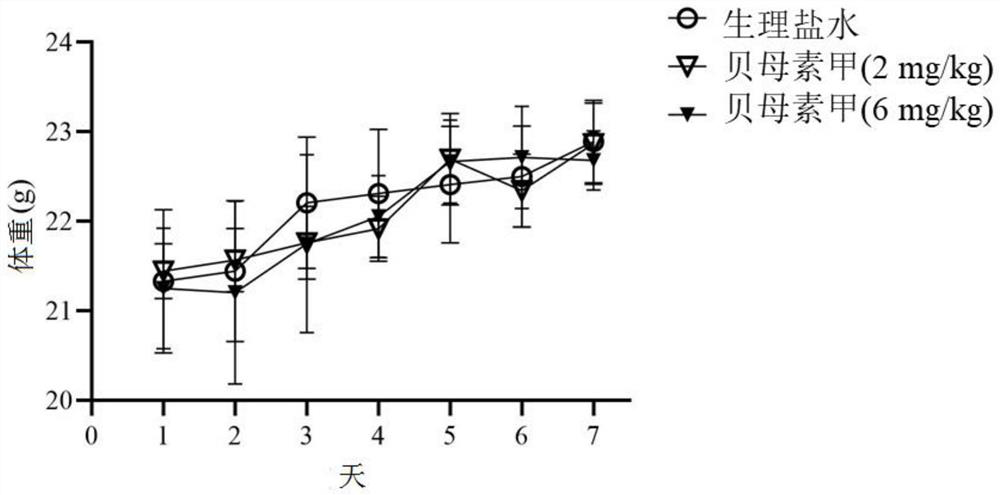
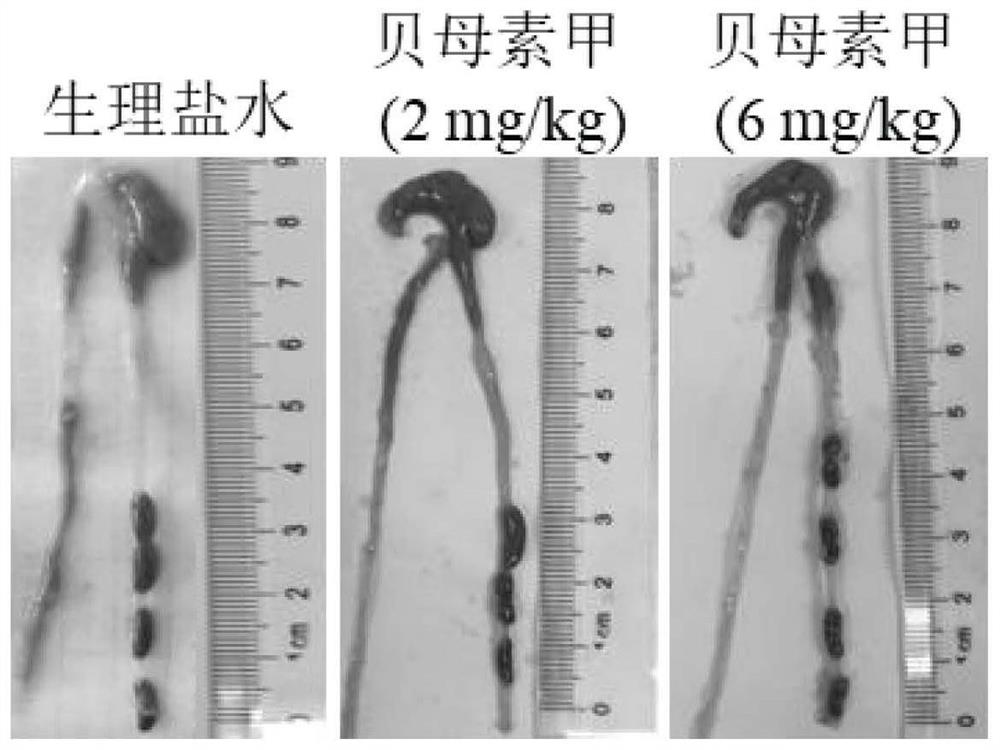
[0049]Eighteen healthy male C57BL / 6J mice with a body weight of about 20 g were randomly divided into 3 groups, 6 in each group, namely the control group, the peimin A (2mg / kg) group and the peimin A (6mg / kg) group. Group. After adapting to the environment for one week, prepare peimin A with physiological saline, and administer it according to the body weight of the experimental mice every day. Perimrin A (6 mg / kg) group was administered 6 mg / kg of peimin A by intragastric administration, and the control group was administered intragastrically with 100 μ L of normal saline for 7 days. At the same time, the death, body weight, intestinal tissue, Cases of colon and cecum pathology.
[0050] Experimental results such as figure 1 , figure 2 , image 3 and Figure 4 shown. The results showed that the administration of peiminine did not cause significant impact on the experimental mice, that is, compared with the normal sali...
Embodiment 2
[0051] The influence of embodiment 2 peiminine A on the disease symptoms of ulcerative colitis mice
[0052] Fifty healthy male C57BL / 6J mice weighing about 20 g were randomly divided into 5 groups, 10 in each group, namely control group, model group, positive drug group, peimin A (2 mg / kg) group and peimin A (6mg / kg) group. After adapting to the environment for one week, the mice were administered daily according to their body weight. The control group drank normal water and administrated 100 μL of normal saline. / kg, the peiminin (6mg / kg) group was given 6mg / kg by intragastric administration of peiminin A, the model group was given intragastric administration of 100 μ L of normal saline, and the positive drug group was given 5-aminosalicylic acid by intragastric administration of 150mg / kg. kg, continuous administration for 7 days. After administration on the first day, 3% dextran sodium sulfate (Dextran Sulfate Sodium Salt, DSS) was added to the drinking water of the exper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com