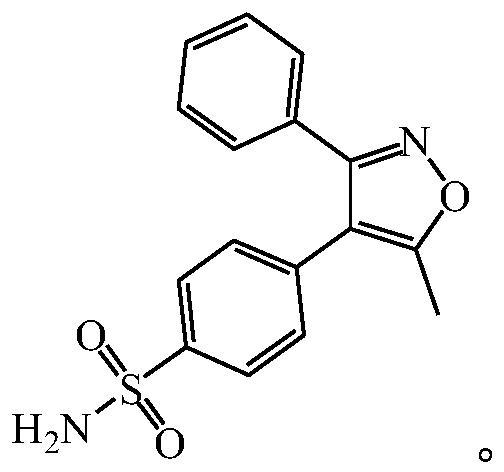
Preparation method of valdecoxib
A technology for valdecoxib and refined products, which is applied in the field of chemical pharmacy, can solve the problems of low purity and yield, unfavorable industrial production of valdecoxib, etc., and achieve the effect of improving purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
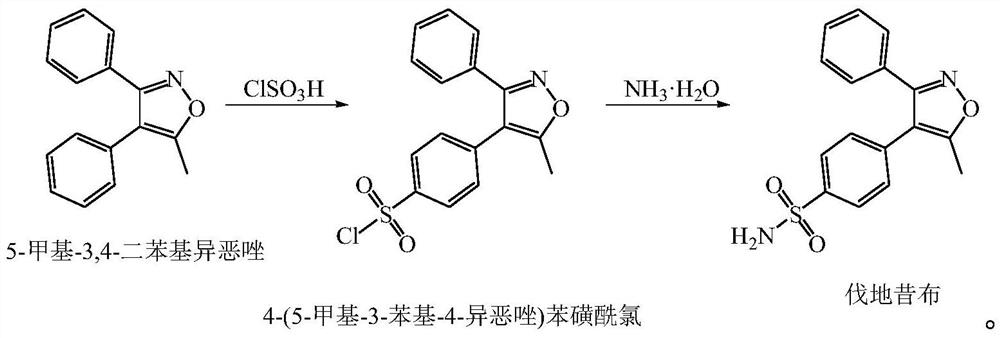
[0046] The present embodiment provides a kind of preparation method of valdecoxib, comprising the following steps:
[0047] (1) Synthesis of 4-(5-methyl-3-phenyl-4-isoxazole) benzenesulfonyl chloride:
[0048] Weigh 19.2kg of dichloromethane and 4.0kg of 5-methyl-3,4-diphenylisoxazole into a 50L glass reactor. Weigh 18.9 kg of chlorosulfonic acid to be added dropwise at a high position. Turn on stirring, cool down in an ice bath, and cool down the temperature of the feed solution to 5°C. Start to add chlorosulfonic acid dropwise, and control the feed liquid temperature <10°C during the dropwise addition. After the addition of chlorosulfonic acid was completed, the temperature was slowly raised to 37° C., and the reaction was kept for 12 hours. Adopt TLC (thin-layer chromatography, developing agent is the mixed solution of ethyl acetate and sherwood oil volume ratio 1:6) monitors to after reaction is complete, the material liquid is left standstill layered, separates the low...
Embodiment 2
[0055] The present embodiment provides a kind of preparation method of valdecoxib, comprising the following steps:
[0056] (1) Synthesis of 4-(5-methyl-3-phenyl-4-isoxazole) benzenesulfonyl chloride:
[0057] Weigh 19kg of dichloromethane and 4.0kg of 5-methyl-3,4-diphenylisoxazole into a 50L glass reactor. Weigh 19kg of chlorosulfonic acid to be added dropwise at a high position. Start stirring, cool down in an ice bath, and cool down the feed solution to 0°C. Start to add chlorosulfonic acid dropwise, and control the feed liquid temperature <10°C during the dropwise addition. After the addition of chlorosulfonic acid was completed, the temperature was slowly raised to 33° C., and the reaction was kept for 14 hours. Adopt TLC (thin-layer chromatography, developing agent is the mixed solution of ethyl acetate and sherwood oil volume ratio 1:6) monitors to after reaction is complete, the material liquid is left standstill layered, separates the lower organic phase layer (4-...
Embodiment 3
[0064] The present embodiment provides a kind of preparation method of valdecoxib, comprising the following steps:
[0065] (1) Synthesis of 4-(5-methyl-3-phenyl-4-isoxazole) benzenesulfonyl chloride:
[0066] Weigh 19.5kg of dichloromethane and 4.0kg of 5-methyl-3,4-diphenylisoxazole into a 50L glass reactor. Weigh 19kg of chlorosulfonic acid to be added dropwise at a high position. Start stirring, cool down in an ice bath, and cool down the temperature of the feed solution to 3°C. Start to add chlorosulfonic acid dropwise, and control the feed liquid temperature <10°C during the dropwise addition. After the chlorosulfonic acid was added dropwise, the temperature was slowly raised to 35°C, and the reaction was kept for 13 hours. Adopt TLC (thin-layer chromatography, developing agent is the mixed solution of ethyl acetate and sherwood oil volume ratio 1:6) monitors to after reaction is complete, the material liquid is left standstill layered, separates the lower organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com