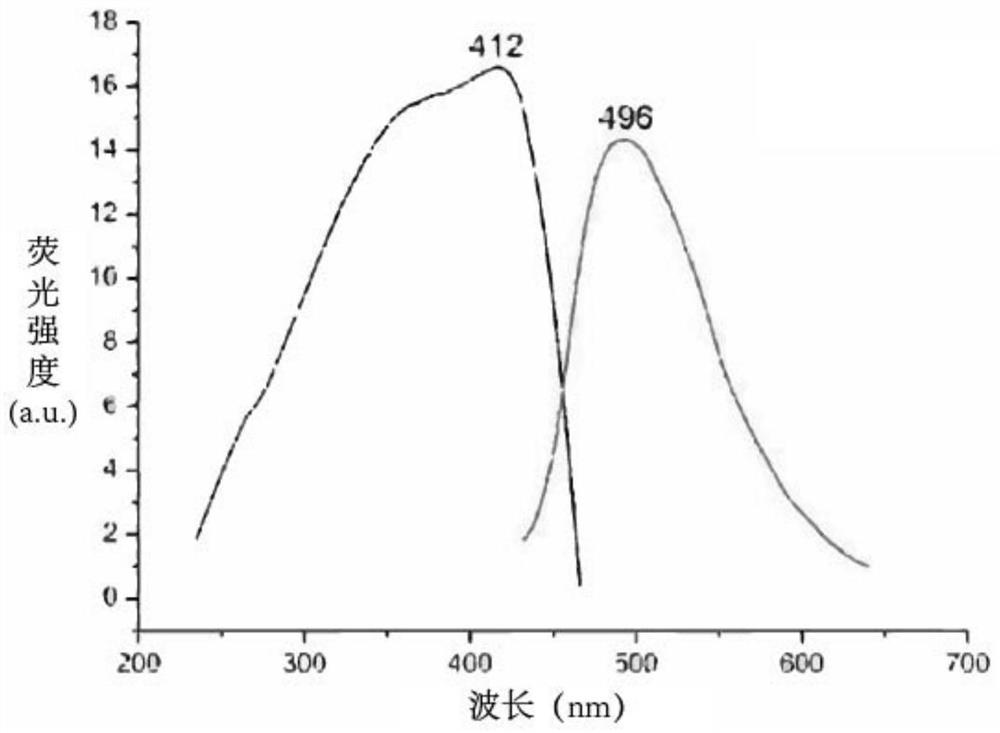
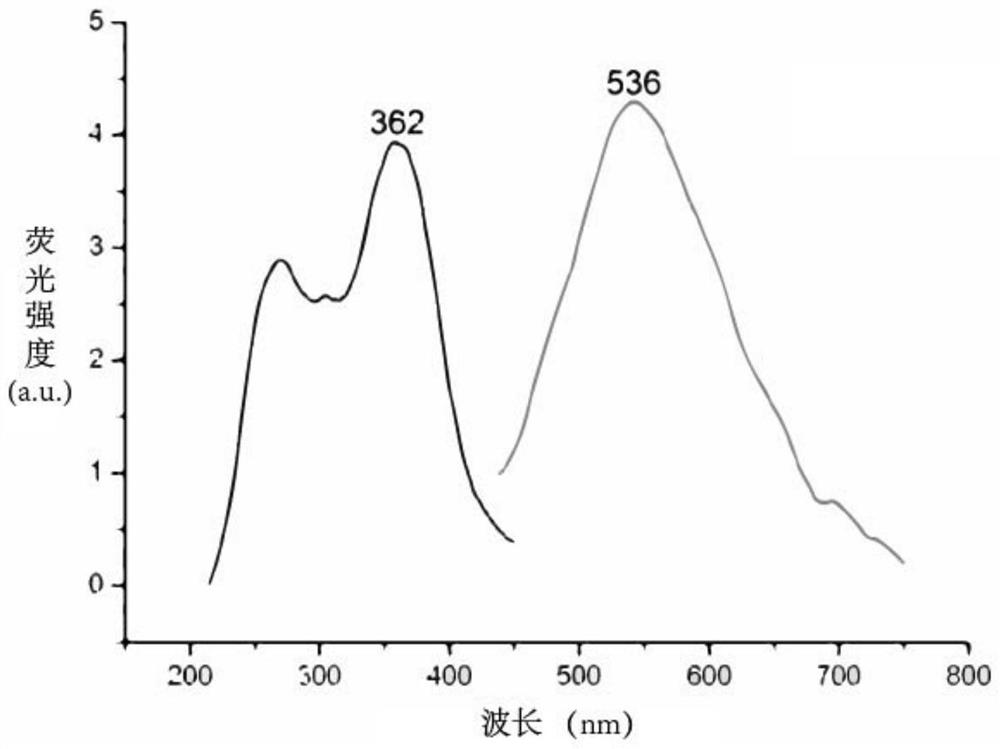
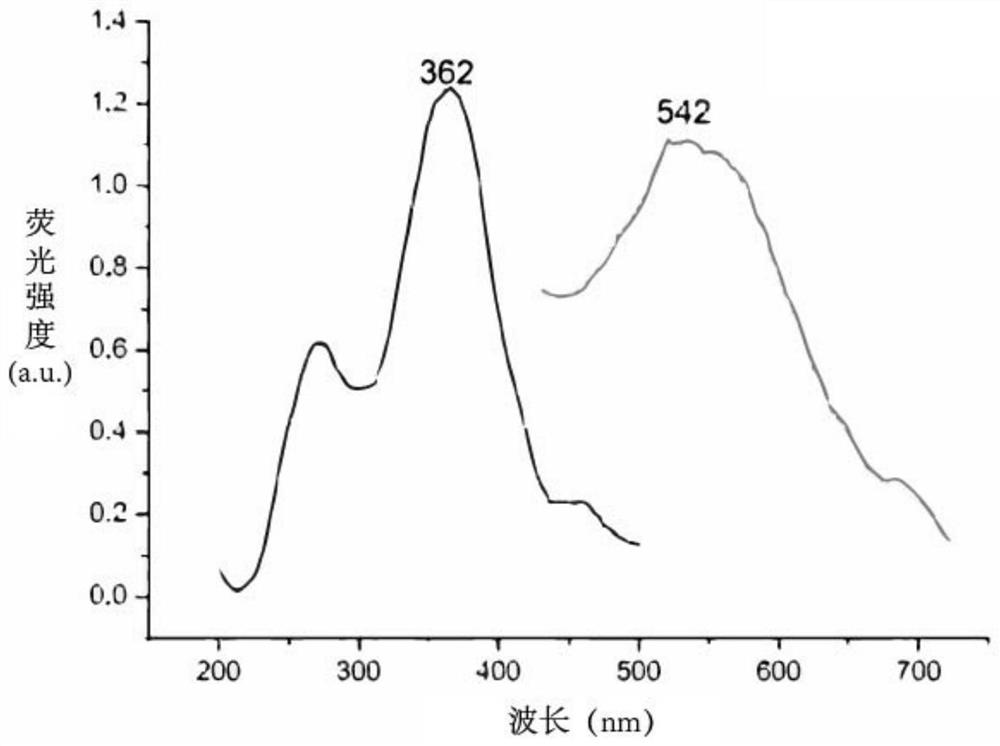
Cell sugar transport channel inhibitor
A sugar transport and inhibitor technology, applied in the field of biomedicine, to achieve the effect of inhibiting tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of GLUT inhibitor (I)-(A-1)-1:
[0042] 1. Synthesis of 4-chloro-6-nitro-7-acetamidobenzo-2-oxa-1,3-oxadiazole (II), the synthetic route is as follows:
[0043] 4-Chloro-7-acetamidobenzo-2-oxa-1,3-oxadiazole (XIV, 678.3 mg, 3.0 mmol) was dissolved in 12 mL of acetic anhydride. With stirring at 0 °C, 4 mL of concentrated nitric acid was slowly added dropwise to the mixture. And after stirring was continued at 0° C. for 3 hours, ice water was added. After stirring for 10 min, it was filtered, and the filter cake was washed 3 times with cold water to obtain 753.3 mg of yellow solid (II), with a yield of 59%. 1 HNMR(600MHz,DMSO-d6):δ11.51(s,1H),8.19(s,1H),2.19(s,3H).
[0044] The reaction formula is:
[0045]
[0046] 2. Synthesis of 4-chloro-6-nitro-7-aminobenzo-2-oxa-1,3-oxadiazole (Ⅲ), the synthetic route is as follows:
[0047] Add Ⅱ (256.6mg, 1.0mmol) to 50%wt.H 2 SO 4 aqueous solution (3 mL), and the mixture was stirred at 100°C for 2 hours. The m...
Embodiment 2
[0066] Synthesis of GLUT inhibitor (I)-(A-2)-1:
[0067] 1, Synthesis of 3-tert-butyldimethylsilyloxybenzoyl chloride (X):
[0068] 3-Hydroxybenzoic acid (VIII, 3.0g, 21.7mmol), tert-butyldimethylsilyl chloride (TBSCl) (7.5g, 50.0mmol) and imidazole (3.4g, 50.0mmol) were dissolved in anhydrous N,N - in dimethylformamide (DMF) (30 mL), stirred at room temperature for 24 h. After the reaction was complete, acetone (150 mL) was added to the reaction mixture, filtered, the filtrate was collected, concentrated under reduced pressure, the resulting residue was dissolved in ethyl acetate (50 mL), washed 3 times with saturated aqueous ammonium chloride, anhydrous sodium sulfate After drying, the solvent was removed under reduced pressure to give a colorless liquid.
[0069] The obtained colorless liquid was dissolved in a mixed solvent of glacial acetic acid (50 mL), tetrahydrofuran (15 mL) and water (15 mL), and stirred at room temperature for 4 h. Ethyl acetate (60 mL) and water ...
Embodiment 3
[0078] Synthesis of GLUT inhibitor (I)-(A-3)-1:
[0079] 1. Synthesis of 3,5-bis(tert-butyldimethylsilyloxy)benzoyl chloride (XIII):
[0080] 3,5-dihydroxybenzoic acid (XI, 3.1 g, 20.1 mmol), tert-butyldimethylsilyl chloride (TBSCl) (10.6 g, 70.4 mmol) and imidazole (4.8 g, 70.4 mmol) were dissolved in anhydrous N,N-dimethylformamide (DMF) (30 mL), stirred at room temperature for 24 h. After the reaction was complete, acetone (150 mL) was added to the reaction mixture, filtered, the filtrate was collected, concentrated under reduced pressure, the resulting residue was dissolved in ethyl acetate (50 mL), washed 3 times with saturated aqueous ammonium chloride, anhydrous sodium sulfate After drying, the solvent was removed under reduced pressure to give a colorless liquid.
[0081] The obtained colorless liquid was dissolved in a mixed solvent of glacial acetic acid (50 mL), tetrahydrofuran (15 mL) and water (15 mL), and stirred at room temperature for 4 h. Ethyl acetate (60 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com