Mycoplasma pneumoniae drug sensitivity rapid detection kit and preparation method thereof
A technology of Mycoplasma pneumoniae and a detection kit, which is applied in the field of clinical medical detection, can solve the problems of inability to accurately judge Mycoplasma pneumoniae infection, a long incubation period of Mycoplasma pneumoniae infection, and no therapeutic effect of Mycoplasma pneumoniae, so as to promote the growth of Mycoplasma pneumoniae and avoid blind drug use. and the effect of overdose and shortened color development time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
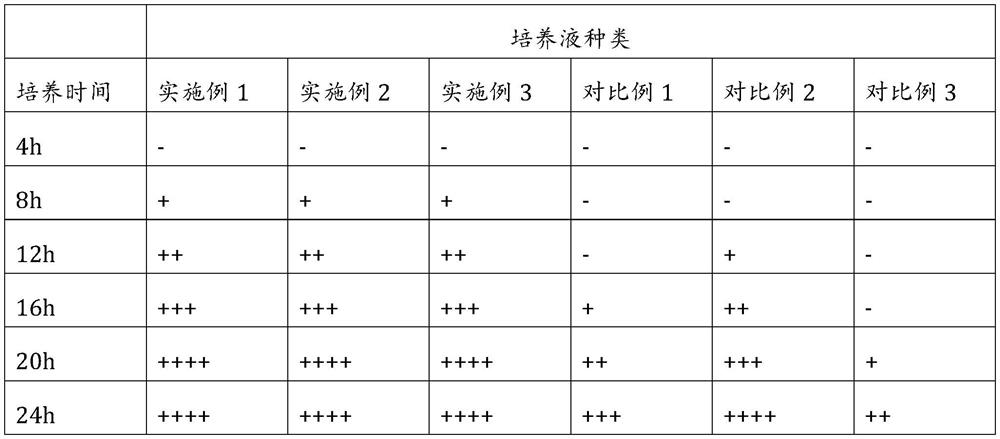
[0027] A rapid detection kit for drug susceptibility to mycoplasma pneumoniae, comprising a mycoplasma pneumoniae culture solution, a drug susceptibility plate and medicines for treating mycoplasma pneumoniae.
[0028] The Mycoplasma pneumoniae culture solution includes the following components: agar powder 9g, peptone 7g, short chain peptide Ala-Asn-Leu 2.6g, morroniside 780mg, yeast powder 4g, glucose 3g, sodium pyruvate 3g, MEM medium 100mL , 2 mL of 1% (w / v) phenol red solution, 150 mL of horse serum, 300,000 units of penicillin and 800 mL of distilled water.
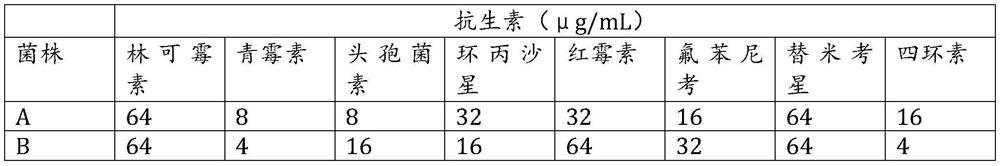
[0029] The drug-sensitivity plate is a 96-well culture plate marked with numbers A to H, and numbers A to H are coated with 8 different drugs in sequence. In wells 1 to 12 of each number, the concentration of the drugs is according to 128μg / mL, 64μg / mL, 32μg / mL, 8μg / mL, 4μg / mL, 2μg / mL, 1μg / mL, 0.5μg / mL, 0.25μg / mL, 0.125μg / mL, 0μg / mL are set in descending order.
[0030] The medicine is the following antibiotic medi...
Embodiment 2
[0038] The short-chain peptide is Ala-Ser-Asn-Thr, and other conditions are the same as in Example 1.
Embodiment 3
[0040] The short-chain peptide is Ala-Ser-Asn-Leu-Val, and other conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com