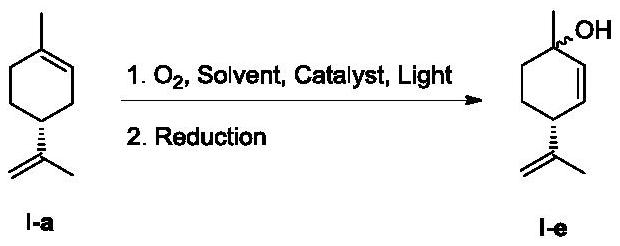
Method for continuously preparing cannabidiol intermediate through green photooxidation
A technology of cannabidiol and a synthesis method, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problem of high comprehensive process cost, and achieve the effects of relatively low cost, environmental friendliness, and effective synthesis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
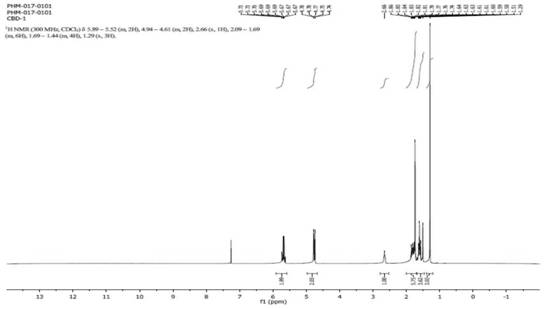
[0039]Add (R)-(+)-limonene (6.80g, 49.92mmol, 1.0eq), acetonitrile (180mL, 26.5V) and catalyst (126mg, 0.12mmol) sequentially into a 250mL glass one-necked bottle, and stir to dissolve. The reaction solution was pumped into the photochemical reactor using a convective pump, and oxygen was continuously fed in at the same time, irradiated continuously with a tungsten halogen lamp (250W) for 18 hours, sampling was controlled, and the residual raw material was 6.9% (GC, area%). The reaction solution was concentrated under reduced pressure to 100 mL, and an aqueous solution of sodium sulfite (12.58 g, 2.0 eq, 15%) was slowly added under ice-cooling, and stirred while adding. After the dropwise addition, a large amount of solid precipitated out. Remove the ice-water bath, continue to stir at room temperature for 2 hours, then raise the temperature to reflux, and keep stirring for 2 hours. Cool down to 40-50°C and concentrate under reduced pressure to remove acetonitrile, add water ...
Embodiment 2
[0041] Add (R)-(+)-limonene (6.80g, 49.92mmol, 1.0eq), acetonitrile (200mL, 29.4V) and catalyst (126mg, 0.12mmol) sequentially into a 250mL glass one-necked bottle, and stir to dissolve. The reaction solution was pumped into the photochemical reactor using a parallel flow pump, and oxygen was continuously fed in at the same time, and the tungsten halogen lamp (500W) was continuously irradiated for 20 hours, and the sampling was controlled, and the residual raw material was 4.2% (GC, area%). The reaction solution was concentrated under reduced pressure to 100 mL, and an aqueous solution of sodium sulfite (12.58 g, 2.0 eq, 15%) was slowly added under ice-cooling, and stirred while adding. After the dropwise addition, a large amount of solid precipitated out. Remove the ice-water bath, continue to stir at room temperature for 2 hours, then raise the temperature to reflux, and keep stirring for 2 hours. Cool down to 40-50°C and concentrate under reduced pressure to remove acetoni...
Embodiment 3
[0043] Add (R)-(+)-limonene (3.40g, 24.96mmol, 1.0eq), acetonitrile (700mL, 205V) and catalyst (63mg, 0.06mmol) to a 1000mL glass one-necked flask in sequence, and stir to dissolve. The reaction solution was pumped into the photochemical reactor using a convection pump, and oxygen was continuously fed in at the same time, irradiated continuously with a tungsten halogen lamp (500W) for 36 hours, the sampling was controlled, and the residual raw material was 8.2% (GC, area%). The reaction solution was concentrated under reduced pressure to 100 mL, and an aqueous solution of sodium sulfite (12.58 g, 2.0 eq, 15%) was slowly added under ice-cooling, and stirred while adding. After the dropwise addition, a large amount of solid precipitated out. Remove the ice-water bath, continue to stir at room temperature for 2 hours, then raise the temperature to reflux, and keep stirring for 2 hours. Cool down to 40-50°C and concentrate under reduced pressure to remove acetonitrile, add water ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com