Pharmaceutical composition for treating chronic liver injury and application of pharmaceutical composition
A technology of chronic liver injury and composition, applied to the pharmaceutical composition for treating chronic liver injury and its application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
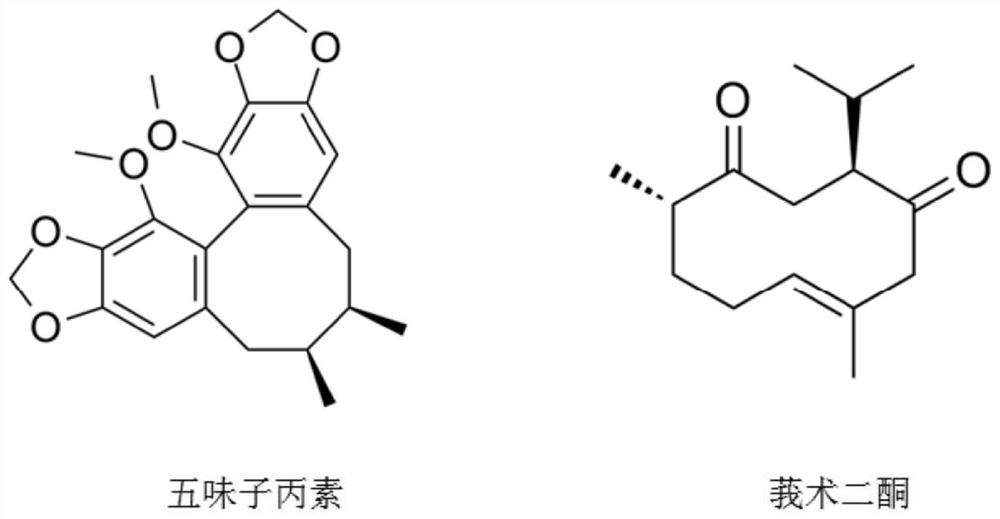
[0049] 1. Preparation of schisandra acetaminophen and curcumadione for cells: take appropriate amount of powder and dissolve them in DMSO respectively to make the final concentration of the mother solution 40mM, and place it at -20°C for later use. Dilute it with medium to dilute with different concentrations when used ( 0-40 μM).
[0050] 2. Preparation of Schisandra C and Curdione for animals: Weigh appropriate amount of Schisandra C and Curdione powder respectively, and make 200mg / kg and 50mg / kg of them with 5% sodium carboxymethyl cellulose solution. The solution for intragastric administration is prepared and used immediately.
[0051] 3. Preparation of APAP solution for cells: Take an appropriate amount of powder and dissolve it in the medium to make the concentration 20mM / L, and store it in the refrigerator at 4 degrees for later use.
[0052] 4. Preparation of each antibody protein: Dilute each antibody protein at a ratio of 1:1000, add sodium azide at a ratio of 1:10...
Embodiment 1
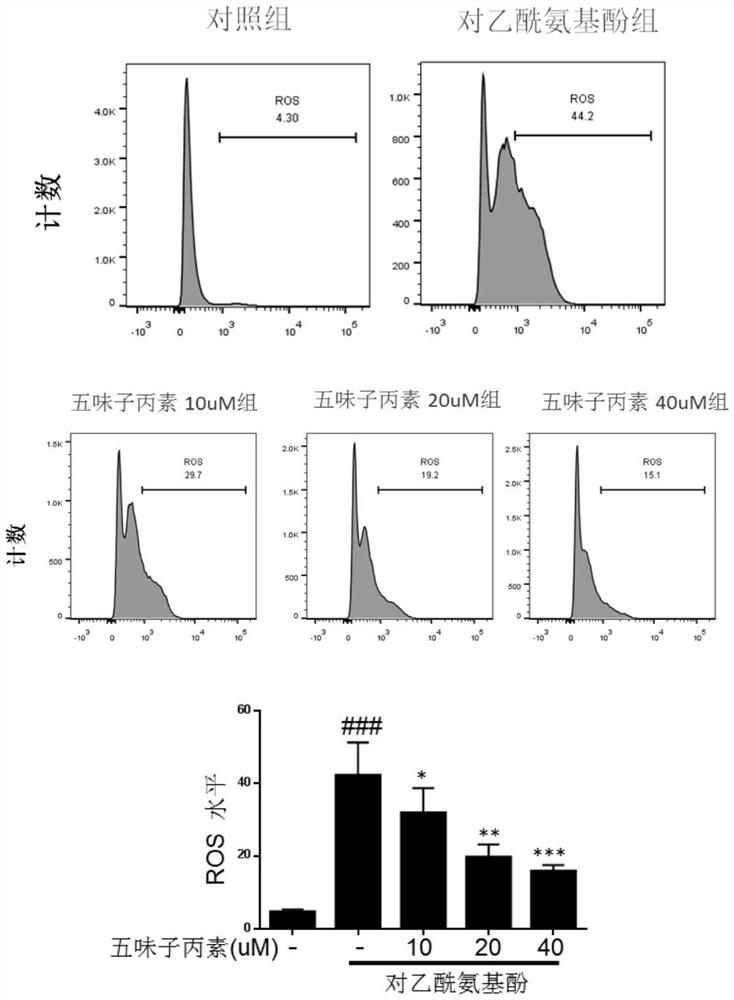
[0056] Inhibitory effect of schisandrin on APAP-induced oxidative stress in Hep G2 cells
[0057] Experimental procedure: Hep G2 cells after passage were divided into 1.5×10 5 cells / ml kind of 12-well plate, with a volume of 1ml per well, divided into blank wells and drug-treated wells, and after the second day’s wall-attachment was successful, the compound Schisandra C (0, 10, 20, 40uM) was added to the drug-treated wells respectively. ) 800ul for 6h (blank wells and control wells were added with an equal volume of drug diluent), and then 20Mm / L of APAP solution was added in the form of liquid exchange (blank wells were replaced with an equal volume of medium) for 12h. After gently aspirating the supernatant, add 150ul of trypsinized cells to each well, transfer to a new EP tube after digestion at 37°C for 1min, centrifuge at 1500rpm, discard the supernatant and wash with HBSS, then add the prepared MitoSOX TM The dye solution was incubated in a 37°C incubator for 15 minutes...
Embodiment 2
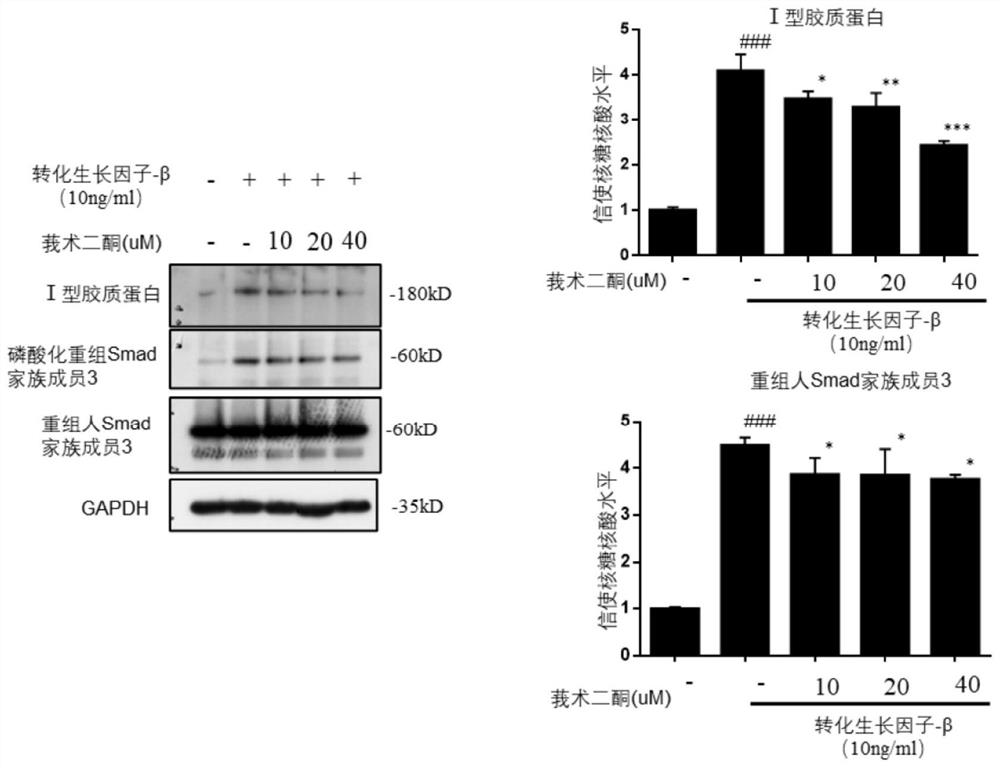
[0060] Specific regulatory effect of curcumadione on TGF-β / smad signaling pathway in LX-2 cells
[0061] Experimental procedure: the passaged LX-2 cells were divided into 2.5×10 5 cells / ml kind of 24-well plate with a volume of 500ul per hole, divided into blank wells and drug-treated wells, and the drug-treated wells were added with different concentrations of compound curcumadione (0, 10, 20, 40uM ) 500ul for 1h (add equal volume of drug diluent to blank wells and control wells), and then add 10ug / ml TGF-β stimulating factor to each well (add equal volume of medium to blank wells) for 24h. After gently aspirating the supernatant, add 150ul of 1×loading RIPA lysate to each well to lyse the cells. After scraping the cell lysate, put it in a metal bath at 105°C for 15min. The protein expression levels of TGF-β / smad signaling pathway-related proteins Collagen, α-SMA, and smad3 were detected by immunoblotting.
[0062] The subcultured LX-2 cells were seeded and treated in the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com