TGF Beta INHIBITOR AND PRODRUGS
An inhibitor, prodrug technology, applied in the treatment of diseases in response to TGFβ signaling inhibition, the field of novel TGFβ signaling pathway inhibitors, can solve problems such as toxicity to healthy tissues and cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Example 1: Synthesis of compounds of formulas I, III and IV:
[0245] Unless otherwise stated, all reactions were performed under an inert atmosphere (N 2 ) under. Anhydrous THF, DCM and ether were obtained from Innovative Technology Inc. Puresolv Solvent Purification System (SPS). Additional anhydrous solvents were purchased and used without any further purification or drying.
[0246] All experiments were monitored by analytical thin layer chromatography (TLC) using silica gel TLC-aluminum plates (Merck 60 F254). Results were visualized under UV light (254 or 365 nm) and displayed using KMnO4 staining if necessary.
[0247] Unless otherwise stated, flash column chromatography was performed on (Teledyne Isco) or on a Puriflash 430 (Interchim). The mobile phase was a gradient of hexane / ethyl acetate or dichloromethane / methanol. In some cases where basic nitrogen was present on the molecule to be purified, the column was preconditioned with 2.5% triethylamine in h...
Embodiment 2
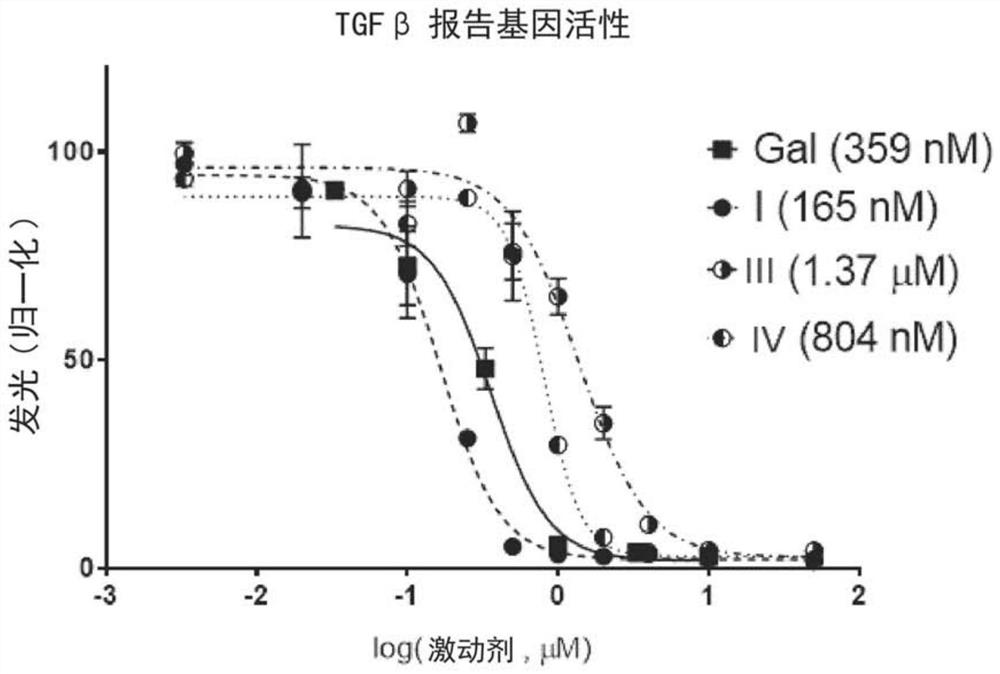
[0318] Example 2: In vitro TGFβ inhibitor activity
[0319] 2.1 Molecular affinity and selectivity determination
[0320] By using KINOMEscan from Eurofins TM techniques to determine the affinity of compounds of formula I for the ALK family, measured as the Kd (dissociation constant). Selectivity to ALK1 (ACVRL1), ALK2 (ACVR1), ALK3 (BMPR1A), ALK4 (ACVR1B), ALK6 (BMPR1B), ACVR2B and TGFβR2 was determined.
[0321] The KINOMEscan screening platform employs an active site-directed competition binding assay to quantitatively measure the interaction between a test compound and a kinase. The KINOMEscan assay does not require ATP and thus reports true thermodynamic interaction affinities.
[0322] The assay is performed by combining three components: DNA-labeled kinase; immobilized ligand; and test compound. The ability of test compounds to compete with immobilized ligands was measured via quantitative PCR of DNA tags.
[0323]Binding reactions were assembled by combining kinas...
Embodiment 3
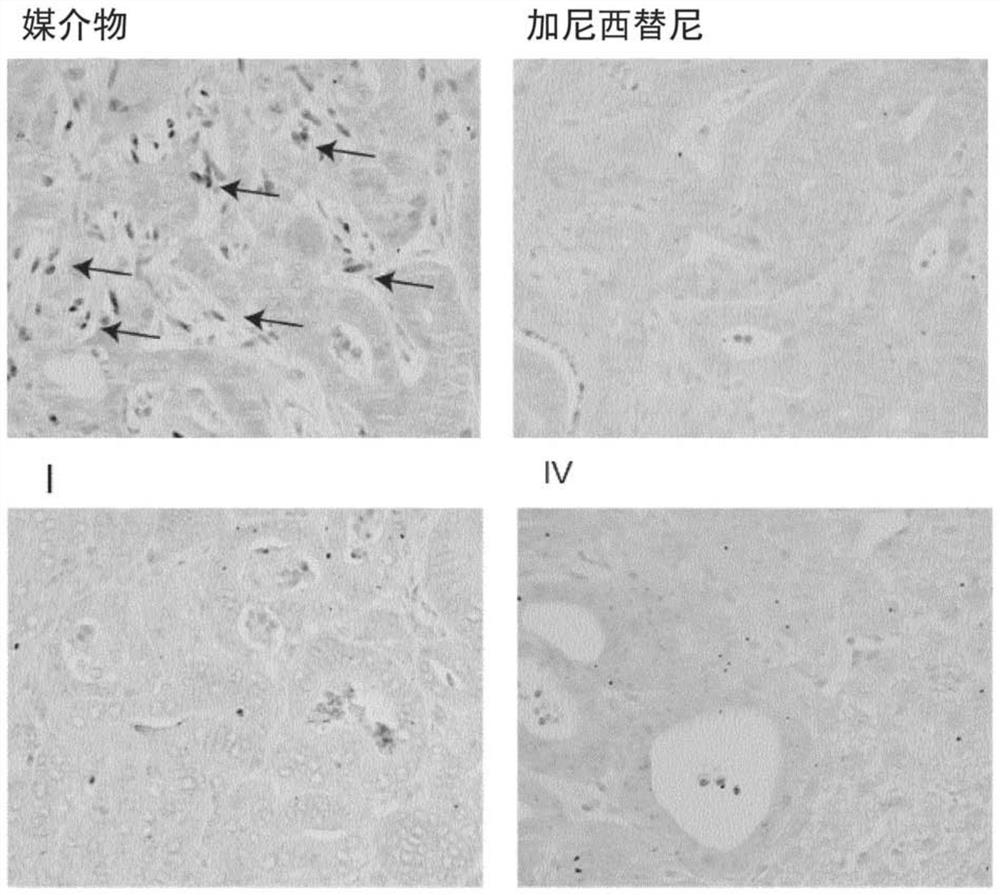
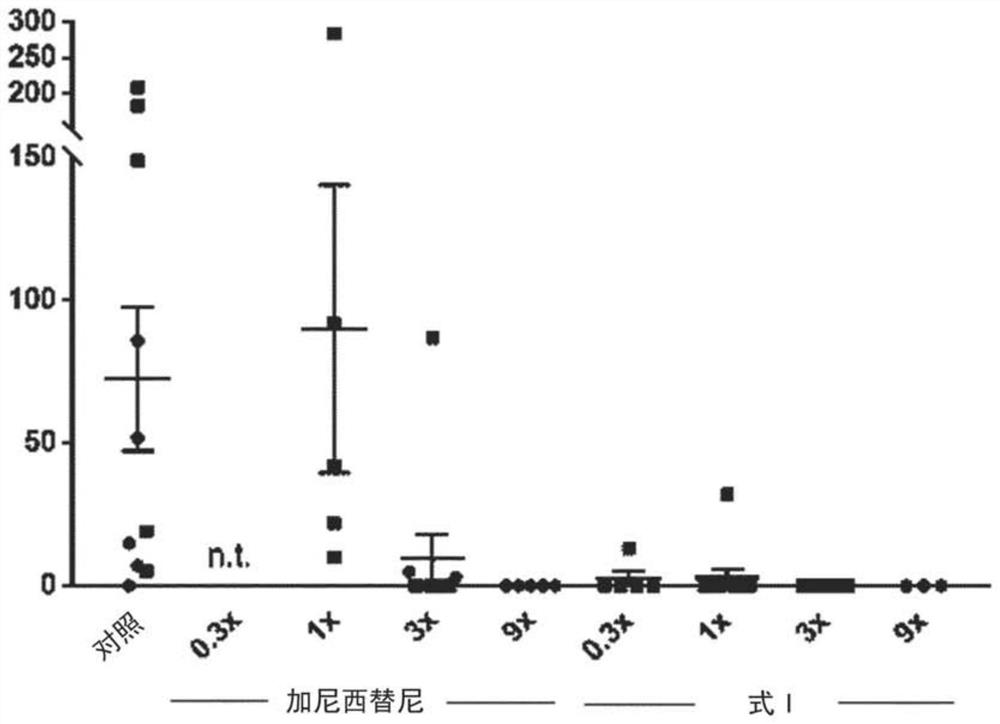
[0345] Example 3: In vivo studies of TGFβ inhibitors
[0346] Mouse tumor organoids (MTOs) were derived from compound transgenic mice of the C57BL6 / J strain (conditional mutations in Apc, Kras, Trp53, and Tgfbr2, and carrying Lgr5-EGFP / CreER T2 ), and cultured as described in Tauriello et al. 2018 Nature 554(7693):538-543.doi:10.1038 / nature25492. Organoids are tumor epithelial cells grown in a 3D matrix in which they adopt organotypic structures, typically as spheroids with lumens. In this way, cells maintain a relatively normal polarity (meaning that the outer surface, inner / luminal surface, and cell-cell contacts are individually specialized), which is more physiological than conventional 2D cell culture. Furthermore, these organoids are able to recapitulate the complex tumor architecture that recapitulates human cancers, including the recruitment of abundant and tumor-promoting (i.e., TGFβ-rich) stroma; a system for engrafting human-like tumors and studying liver metastase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com