Alltrenogest sustained-release injection and preparation method and application thereof
A technology for allyrogestin and injection, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and medical preparations without active ingredients, etc. Sexual issues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of allyl progesterone injection
[0022] An allylgestrine injection, the preparation method of which comprises: weighing dimethyl sulfoxide and sucrose acetate isobutyrate, and mixing them at a mass ratio of 4:1 at 27°C to obtain a clear, precipitate-free, transparent solvents such as figure 1 As shown, then add allylgestrine, the preparation obtains the allylgestrine content that is 160mg / mL clarification does not have the allylgestrine injection of precipitation, as figure 2 shown.
Embodiment 2
[0023] Embodiment 2: the mensuration of release degree in vitro
[0024] (1) First prepare 0.01mol / L PBS buffer solution of pH7.4, mix 1.6gNaCl, 0.04g KH 2 PO 4 , 0.58gNa 2 HPO 4 , 0.04g KCl was added to the beaker, dissolved with a certain amount of distilled water, then transferred to a 200mL volumetric flask for constant volume, and finally the pH of the solution was adjusted to 7.4 with HCl.
[0025] Take 5mL of absolute ethanol into a 10mL volumetric flask, and then dilute with PBS buffer to prepare 50% ethanol PBS buffer.
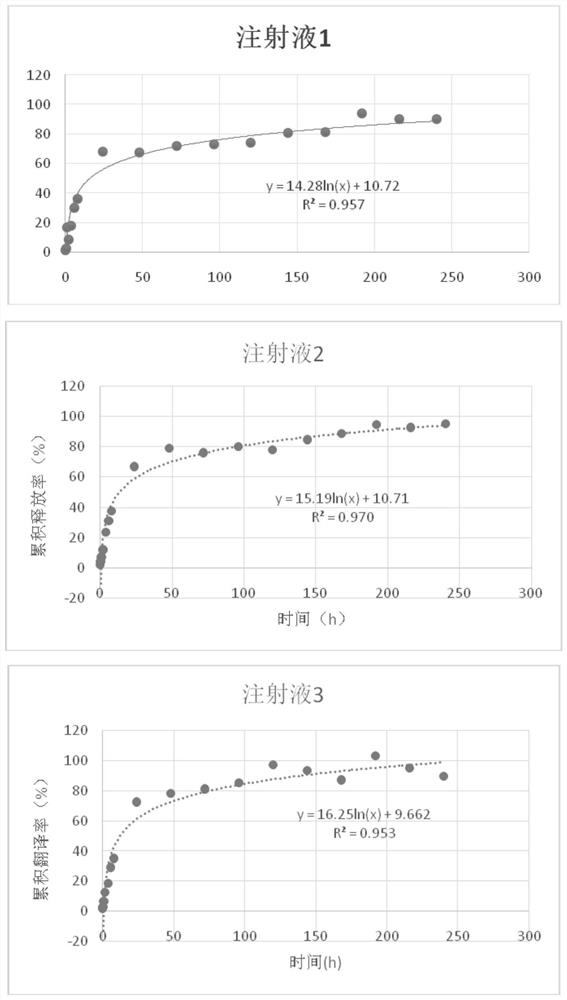
[0026] (2) Three parts (injections 1, 2, 3) of the allylgestrine injection in Example 1 (the content of allylgestrine is 160 mg / mL), one part without allylgestrine and only dimethyl The blank injection of sulfoxide and sucrose acetate isobutyrate (dimethyl sulfoxide and sucrose acetate isobutyrate were mixed at a mass ratio of 4:1 at 27°C to obtain a clear solution without precipitation, transparent solvent), a part of dimethyl sulfoxide solution...
Embodiment 3
[0035] Embodiment 3: the mensuration of recovery rate and stability
[0036] (1) Preparation of standard curve
[0037] Prepare a solution of allylregestin absolute ethanol with a concentration of 1 mg / mL as the mother liquor, and dilute the mother liquor into 100 μg / mL, 80 μg / mL, 60 μg / mL, 40 μg / mL and 20 μg / mL of allylregestin absolute ethanol standard solution. Using absolute ethanol as a blank, measure the absorbance of the standard solution of each concentration respectively, and record it well, and make a standard curve. The results are as follows: Figure 4 As shown, the correlation coefficient r 2 >0.99, good linear performance.
[0038] (2) determination of content, recovery and stability
[0039] Get 4 samples of allylgestrine sustained-release injection in Example 1 (corresponding to 0-3), be mixed with dehydrated alcohol into 20, 40, 80 μ g / mL allylgestrine solution, measured content, recovery The rates and coefficients of variation are shown in Table 2. It can...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com