Preparation method of dibutylboron trifluoromethanesulfonate
A technology of dibutyl boron trifluoromethanesulfonate and organic solvents, which is applied in the field of medicinal chemistry and can solve the problems of inability to realize large-scale production and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
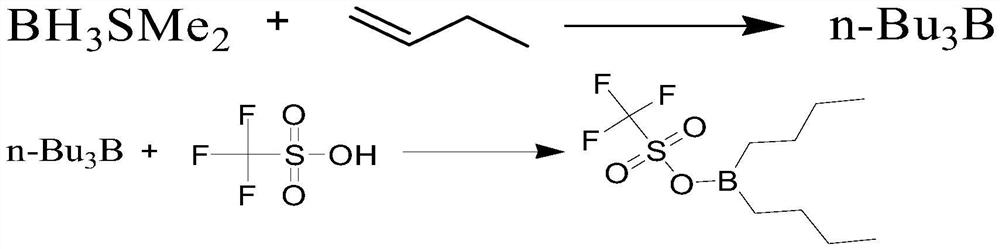
[0034]
[0035] (n-Bu is n-butyl)
[0036] Under an inert nitrogen atmosphere, the BH 3 ·SMe 2 Soluble in an aprotic organic solvent, the aprotic organic solvent is ether, tetrahydrofuran, n-hexane, etc., preferably n-hexane, the temperature of the system is raised to 20-60°C, preferably 45-50°C, and then 10% n-butene hexane The alkane solution is added to the constant pressure dropping funnel, and slowly added dropwise to the system, wherein, the solvent volume selection range: 1 to 10 parts by volume, preferably 3 parts by volume; BH 3 ·SMe 2 The molar ratio to n-butene is 1:3 to 1:10, and this range includes the numerical range of any subrange therein, and non-limiting examples are 1:3 and 1:4. The temperature of the whole reaction process is controlled at 0-60°C, preferably 45-50°C. After the dropwise addition, the temperature of the system is maintained at 45-50°C for 1-8 hours, preferably 1-6 hours. After the reaction is completed, the temperature is lowered to -2...
Embodiment 2
[0038] (n-Bu is n-butyl)
[0039] Under an inert gas nitrogen atmosphere, the n-Bu 3B is dissolved in an aprotic organic solvent, and the aprotic organic solvent is dichloromethane, tetrahydrofuran, n-hexane, etc., preferably dichloromethane, and the temperature of the system is reduced to -20~0°C, preferably -5~0°C, and three Fluoromethanesulfonic acid is dissolved in dichloromethane, added to the constant pressure dropping funnel, and slowly added dropwise to the system, wherein, the solvent volume selection range: 1 to 10 parts by volume, preferably 2 parts by volume; n-Bu 3 The molar ratio of B to trifluoromethanesulfonic acid is 1:1 to 1:10, and this range includes the numerical range of any subrange therein, and non-limiting examples are 1:1 and 1:2. The temperature of the whole reaction process is controlled at -5 ~ 10 ° C, preferably 0 ~ 5 ° C, after the dropwise addition, maintain this temperature for 0 ~ 0.5 hours, then raise the temperature to 20 ~ 40 ° C, prefer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com