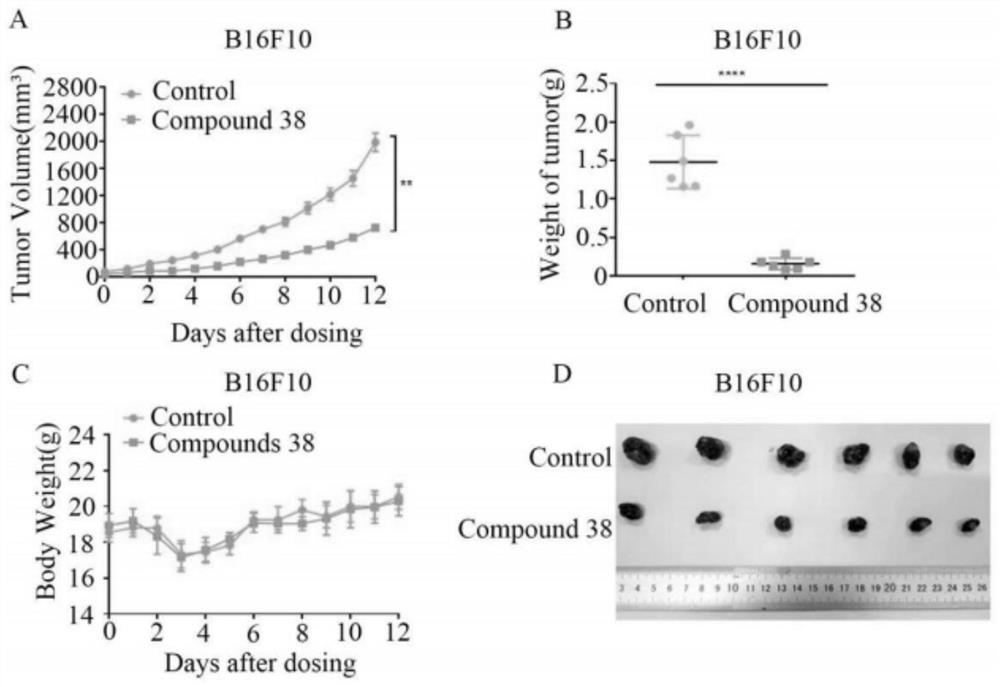
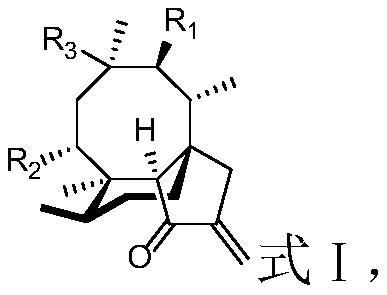
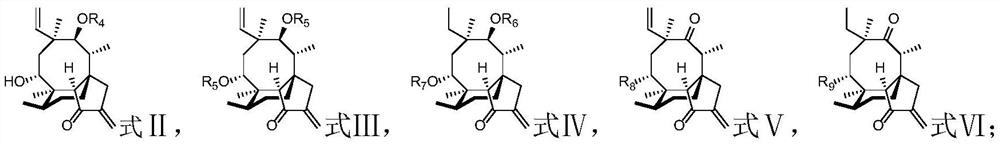
A kind of diterpene derivative and its preparation method, pharmaceutical composition and application
A technology of derivatives and compositions, applied in the field of diterpene derivatives and their preparation, can solve problems such as insufficient killing ability of cancer cells, and achieve the activity of effectively inhibiting the proliferation of various tumor cells, obvious anti-cancer activity, and the preparation process. Ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of diterpene derivatives
[0040] (1) Preparation of compound 3:
[0041] The structure of compound 3 is as follows:
[0042] Its preparation process is as follows:
[0043] To a solution of pleuromutilin 1 (7.00 g, 22.1 B mmoL) in ethanol (50 mL) and water (32 mL) was added 50% aqueous sodium hydroxide (3.70 mL) at room temperature. After stirring at 65°C for 1 hour, the reaction mixture was cooled to room temperature and filtered, and the filtrate was acidified with 1N hydrochloric acid to pH=2, then diluted with ethyl acetate (200 mL); the organic phase was separated, and the aqueous phase was washed with ethyl acetate (3× 200 mL) for extraction; the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, concentrated in vacuo, and used directly for the next step.
[0044] To a solution of diol compound 2 (5.50 g, 17.2 mmol) in N,N-dimethylformamide (60 mL) was added tetramethylmethanediamine (60 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com