Methods and compositions for the treatment of fabry disease
A genome and viral vector technology, applied in the field of gene therapy to prevent and/or treat Fabry disease, can solve problems such as α-GalA level fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0275] High plasma α-Gal A activity persists for 3 months in GLAKO mice treated with variant #4 expression construct
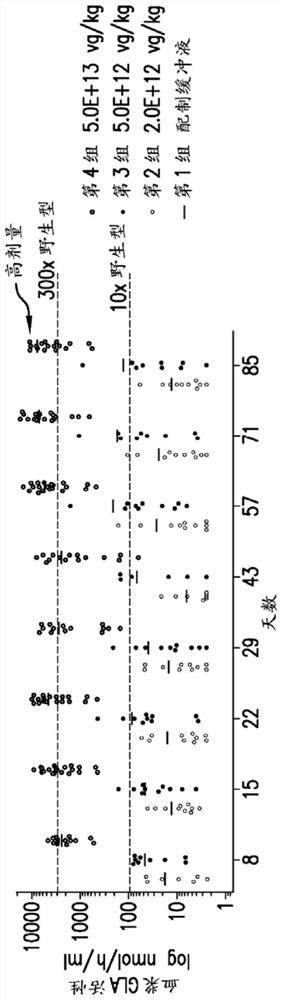
[0276] will be like Figure 1A Samples of the indicated variant #4 expression constructs were administered to male GLAKO mice to evaluate pharmacodynamic activity and biodistribution following a single intravenous (IV) dose.
[0277] GLAKO male mice were 8-12 weeks old at the start of the study. Animals (n=10-20 males / group) received formulation buffer containing phosphate buffered saline (PBS) containing CaCl2, MgCl2, NaCl, sucrose and Kolliphor (poloxa) on day 1. (M)P 188) (control mice)) or variant #4 expression vector receiving one of three dose levels (2.0E+12, 5.0E+12 or 5.0E+13 vg / kg, respectively; n=10 mice per group) as a single 200 μl tail vein administration. Mice were monitored for 3 months. figure 2 The results of the pharmacokinetic evaluation (plasma α-Gal A activity) in individual mice are presented in and image 3 Group means (mean + SD) ...
Embodiment 2
[0281] High levels of α-Gal A activity lead to a corresponding reduction in Fabry substrates
[0282] Variant #4 construct formulations were administered intravenously to GLAKO mice at doses of 0 vg / kg, 2.0E+12 vg / kg, 5.0E+12 vg / kg, or 5.0E+13 vg / kg to evaluate mouse plasma and tissue Fabry substrate levels in . Tissues were harvested at necropsy on day 91 post-dose and levels of the α-Gal A substrate Gb3 (isoforms C22:0 and C24:0) and its deacylated form lyso-Gb3 were determined using LC-MS . Briefly, tissues were weighed and mechanically disrupted in tissue disruption fluid (5% MeOH, 95% water and 0.1% acetic acid) at a rate of 5 ml fluid / mg tissue. Then 10 μl of plasma or tissue slurry was added to 90 μl of precipitation solvent (MeOH with internal standard N-tricosylceramide trihexoside (C23:0, Matrya) spiked into solution) in a siliconized tube, vortexed and placed in Place on a shaking plate at room temperature for 30 minutes. Samples were then centrifuged and 10 μl ...
Embodiment 3
[0287] Variant #21 expression vector produces plasma α-Gal A activity in vitro and in vivo.
[0288] The level and activity of secreted human α-Gal A was assessed in various mouse, cynomolgus monkey, and human primary cells and cell lines following transduction with variant #4 or variant #21 expression vectors. Variant #4 or variant #21 expression vectors were produced in 1) HEK293 cells or 2) Sf9 insect cell line.
[0289] HepG2 cells and iPSC-derived hepatocytes (iCell hepatocytes) were transduced using standard techniques and as described in US Publication No. 20180117181. Briefly, cells were seeded at varying densities per well and transduced at a multiplicity of infection (MOI) ranging from 100,000 to 600,000 vg variant #21 expression construct or variant #4 expression construct per cell. Supernatant samples were collected on days 3 to 7 and α-Gal A enzyme activity was assessed by α-Gal A fluorescence activity assay and in cell pellets collected at the end of the study (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com