Effervescent preparation of compound oral rehydration salt as well as preparation process and application of effervescent preparation
A technology of effervescent preparations and rehydration salts, which is applied in the fields of application, medical preparations containing active ingredients, and pill delivery, which can solve the problems of cumbersome taking procedures, large side effects, and large amount of drinking fluids, so as to improve immunity and improve Intestinal function, the effect of overcoming side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
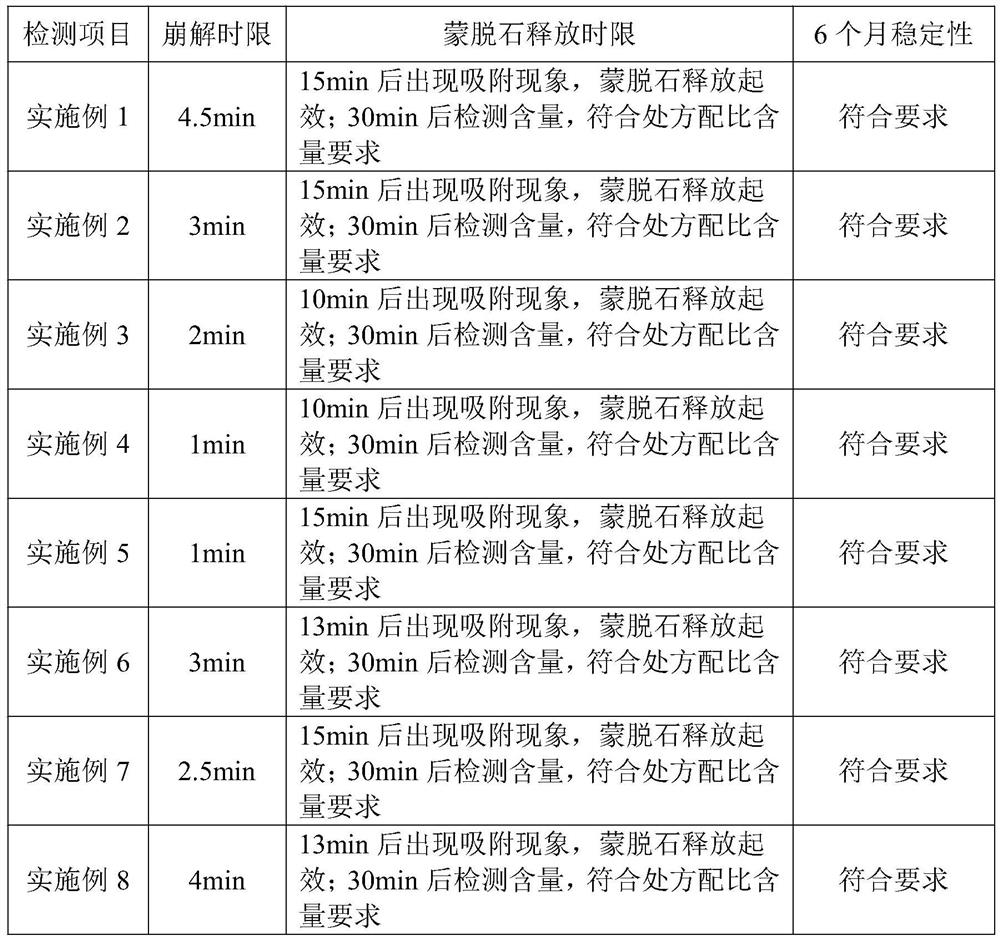
Examples
Embodiment 1
[0060] (1) The formula of the effervescent preparation of compound oral rehydration salt
[0061] The effervescent formulation of this compound ORS includes probiotics 1 x 10 9 CFU / g, 0.1 parts of prebiotics, 10.9 parts of rehydration salts, 58 parts of modified montmorillonite coated granules, and 31 parts of effervescent auxiliary materials. Specifically, it includes the following components;
[0062] Including rehydration salt components: 2.6 parts of sodium chloride, 1.2 parts of potassium chloride, 1.6 parts of citrate, and 5.5 parts of anhydrous glucose.
[0063] Including modified montmorillonite coating particle components: 34.5 parts of montmorillonite, 1.5 parts of sodium pyruvate, 0.5 parts of ethyl cellulose, 0.5 parts of chitosan, 1 part of acetyl ethyl citrate and PVP- K30 is 20 servings.
[0064] Including effervescent auxiliary material components: 5 parts of acid source, 5 parts of alkali source, 14 parts of filler, 2.7 parts of disintegrant, 2 parts of lub...
Embodiment 2
[0086] (1) The formula of the effervescent preparation of compound oral rehydration salt
[0087] The effervescent formulation of this compound ORS includes probiotics 1 x 10 9 CFU / g, 0.5 parts of prebiotics, 15.9 parts of rehydration salts, 48 parts of modified montmorillonite coated granules, and 35.6 parts of effervescent auxiliary materials. Specifically, it includes the following components;
[0088] Including rehydration salt components: 4.5 parts of sodium chloride, 3 parts of potassium chloride, 2.3 parts of citrate, 6.1 parts of anhydrous glucose.
[0089] Including modified montmorillonite coating particle components: 30 parts of montmorillonite, 1 part of sodium pyruvate, 0.5 part of ethyl cellulose, 0.5 part of chitosan, 1 part of acetyl ethyl citrate and PVP- K30 is 15 servings.
[0090] Including effervescent auxiliary material components: 6 parts of acid source, 6 parts of alkali source, 16 parts of filler, 3.3 parts of disintegrant, 2 parts of lubricant, 0...
Embodiment 3
[0112] (1) The formula of the effervescent preparation of compound oral rehydration salt
[0113] The effervescent formulation of this compound ORS includes probiotics 1 x 10 9 CFU / g, 0.5 parts of prebiotics, 20.9 parts of rehydration salts, 39 parts of modified montmorillonite-coated granules, and 39.6 parts of effervescent auxiliary materials. Specifically, it includes the following components;
[0114] Including rehydration salt components: 6.5 parts of sodium chloride, 2.5 parts of potassium chloride, 2.8 parts of citrate, and 8.1 parts of anhydrous glucose.
[0115] Including modified montmorillonite coating particle components: 24 parts of montmorillonite, 0.5 part of sodium pyruvate, 0.5 part of ethyl cellulose, 0.5 part of chitosan, 0.5 part of acetyl ethyl citrate and PVP- K30 is 13 servings.
[0116] Including effervescent auxiliary material components: 7 parts of acid source, 7 parts of alkali source, 19 parts of filler, 3.3 parts of disintegrant, 1 part of lubri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com