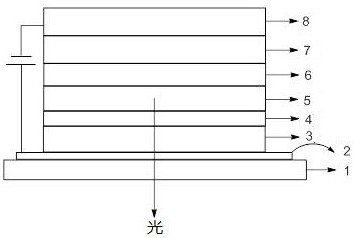
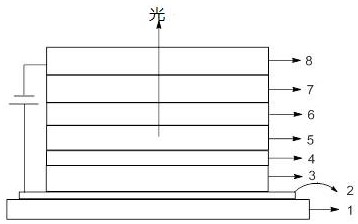
Anthracene imidazole derivative, organic electroluminescent material and consumer product
A technology of anthrazimidazole and its derivatives, which is applied in the field of organic electroluminescent materials, can solve the problems of low thermal stability and high driving voltage, achieve lower starting voltage, increase π-π conjugation intensity, improve luminous efficiency and The effect of brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation method of compound CJHP196 comprises the steps:
[0065] The first step: preparation of intermediate Int-1
[0066]
[0067] 54.5 mmol of 3-iodo-2-cyanonaphthalene (prepared with reference to patent CN104193783A) and 60.0 mmol of phenylacetylene, 5.5 mmol of cuprous iodide, 0.5 mmol of PdCl 2 (PPh 3 ) 2 Catalyst, then add 80 mL of THF and 10 mL of triethylamine, under the protection of nitrogen, stir the reaction at room temperature for 8 hours, filter, concentrate and dry the filtrate under reduced pressure, then separate and purify with silica gel column to obtain Int-1 as a yellow solid, The yield is 94%.
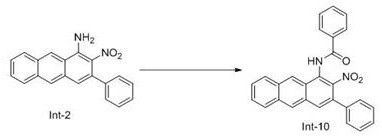
[0068] The second step: the preparation of intermediate Int-2
[0069]
[0070] Dissolve 50.0 mmol of Int-1 in 80 mL of DMSO, add 0.1 mol of nitromethane and 0.1 mol of potassium hydroxide under nitrogen protection, raise the temperature to 110°C and stir for 1 hour, cool to room temperature, add 150 mL Saturated aqueous sodium bisulfite...
Embodiment 2
[0095] With reference to the synthetic method of Example 1, prepare the following compounds, that is, the method steps are the same as in Example 1, the difference is only that according to the desired product, different compounds are used to replace the 3-iodine in the first step of Example 1 according to actual needs -2-cyanonaphthalene and phenylacetylene, replace the 2-naphthylboronic acid in the ninth step of embodiment 1, and change the mass consumption of this compound according to the molar weight, prepare the following compounds:
[0096]
Embodiment 3
[0098] Preparation of Compounds CJHP186, CJHP187, CJHP197 and CJHP198:
[0099] With reference to the synthetic method of Example 1, prepare the following compounds, that is, the method steps are the same as in Example 1, the difference is only that according to the desired product, different compounds are used to replace the 3-iodine in the first step of Example 1 according to actual needs -2-cyanonaphthalene is 6-bromo-3-iodo-2-cyanonaphthalene or 7-bromo-3-iodo-2-cyanonaphthalene, and the mass dosage of the compound is changed according to the molar weight to prepare the compound: CJHP186 , CJHP187, CJHP197 and CJHP198;
[0100] Compound CJHP186, MS(MALDI-TOF): m / z=676.2772 [M+H] + ;
[0101] Compound CJHP187, MS(MALDI-TOF): m / z=676.2726 [M+H] + ;
[0102] Compound CJHP197, MS(MALDI-TOF): m / z=776.3008 [M+H] + ;
[0103] Compound CJHP198, MS(MALDI-TOF): m / z=776.3036 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com