Cobalt disulfide/nitrogen-sulfur co-doped mesoporous carbon composite catalyst for zinc-air battery and preparation method of cobalt disulfide/nitrogen-sulfur co-doped mesoporous carbon composite catalyst
A nitrogen-sulfur co-doping, zinc-air battery technology, applied in fuel cell type half cells and secondary battery type half cells, battery electrodes, circuits, etc. Catalytic activity, simple process, and the effect of preventing metal particle agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
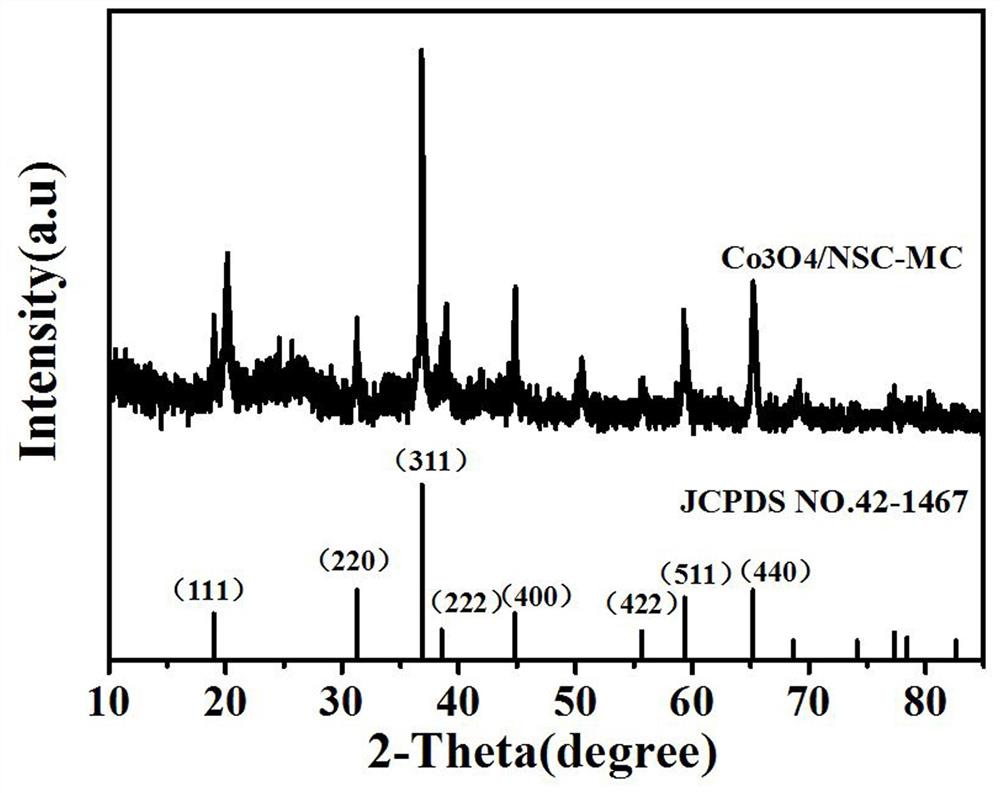
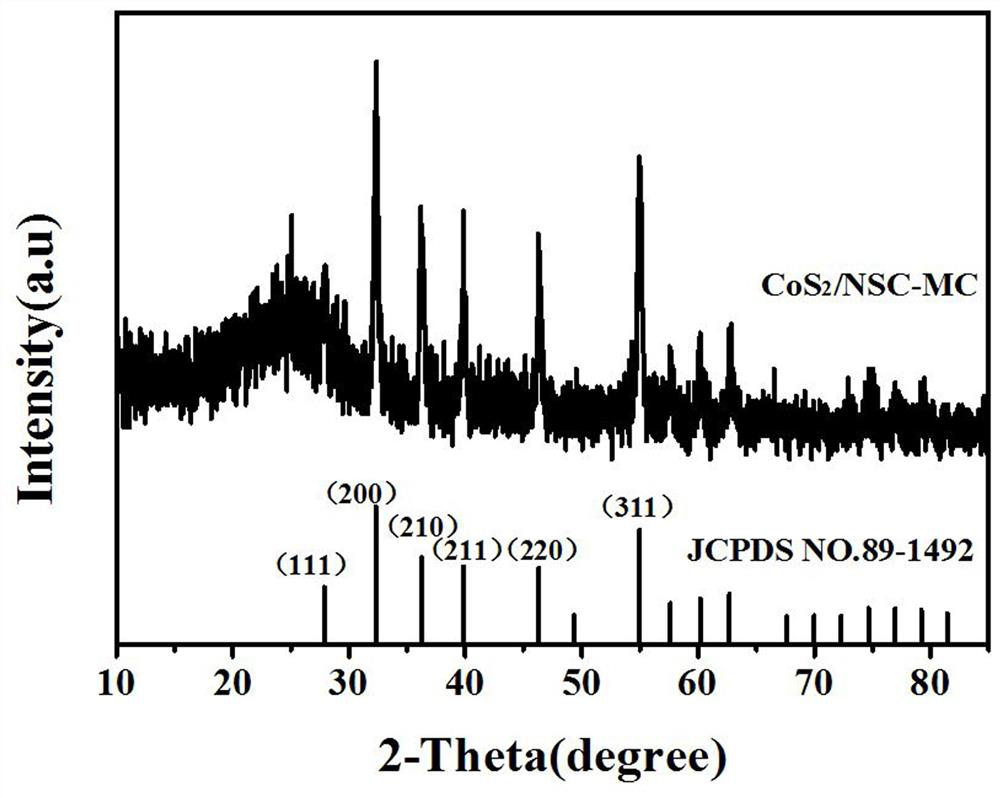
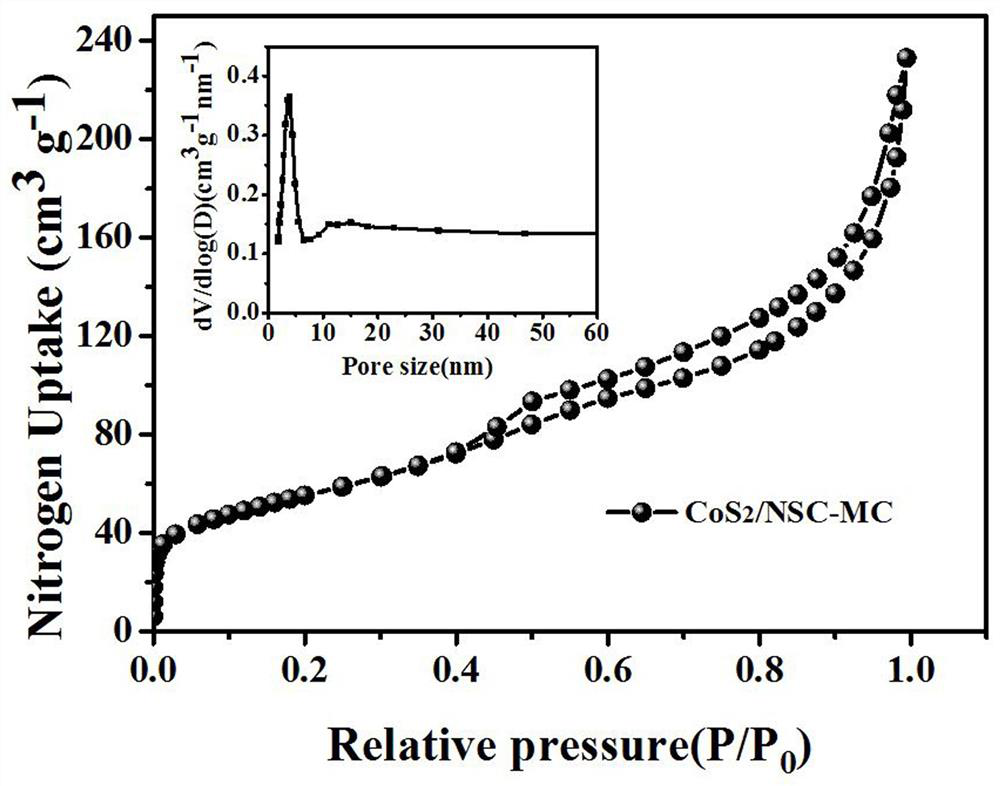
Image
Examples
Embodiment 1
[0038] (1) Weigh 0.3 g of cobalt acetate, 0.989 g of 2-methylimidazole and 0.027 g of β-cyclodextrin, and dissolve them in ethanol and water, respectively. The three solutions were mixed and stirred for 0.5 h.
[0039] (2) Weigh 0.25 g of asphalt and 0.195 g of SBA-15 and put them in an agate mortar, add the mixed solution obtained in step (1), and grind evenly under a heat lamp to fully mix the raw materials with the silica template to obtain Calcined precursors.
[0040] (3) The calcined precursor obtained in step (2) was placed in a tube furnace for calcination under a nitrogen atmosphere. First, the temperature was raised to 300 °C and kept for 2 h, then continued to be heated to 600 °C and kept for 2 h at a heating rate of 5 °C. min-1.
[0041] (4) The calcined product obtained in step (3) was washed with alkali in 1 mol L-1 NaOH solution at 60 °C or 80 °C for 24 h to remove the silica template. In this implementation, alkali washing is preferably performed at 80°C.
...
Embodiment 2
[0045] (1) Weigh 0.3 g of cobalt acetate, 0.989 g of 2-methylimidazole and 0.0068 g of β-cyclodextrin, and dissolve them in ethanol, ethanol and water respectively. The three solutions were mixed and stirred for 0.5 h.
[0046] (2) Same as step (2) in Example 1 of the manufacturing method.
[0047] (3) Same as step (3) in Example 1 of the manufacturing method.
[0048] (4) Same as step (4) in Example 1 of the manufacturing method.
[0049] (5) Same as step (5) in Example 1 of the production method.
[0050] (6) Same as step (6) in Example 1 of the manufacturing method.
Embodiment 3
[0052] (1) Weigh 0.3 g of cobalt acetate, 0.989 g of 2-methylimidazole and 0.0034 g of β-cyclodextrin, and dissolve them in ethanol, ethanol and water respectively. The three solutions were mixed and stirred for 0.5 h.
[0053] (2) Same as step (2) in Example 1 of the manufacturing method.
[0054] (3) Same as step (3) in Example 1 of the manufacturing method.
[0055] (4) Same as step (4) in Example 1 of the manufacturing method.
[0056] (5) Same as step (5) in Example 1 of the production method.
[0057] (6) Same as step (6) in Example 1 of the manufacturing method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com