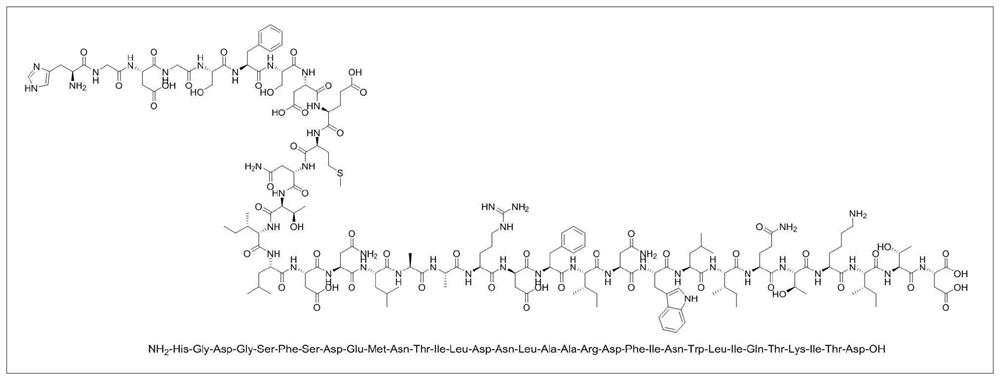
Preparation method of teduglutide
A teduglutide and gradient technology, applied in the field of drug synthesis, can solve the problems of insufficient selection of solid-phase fragments, low overall yield, low purity of crude products, etc., reduce material costs and purification costs, and improve single-batch production Scale, the effect of reducing the difficulty of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
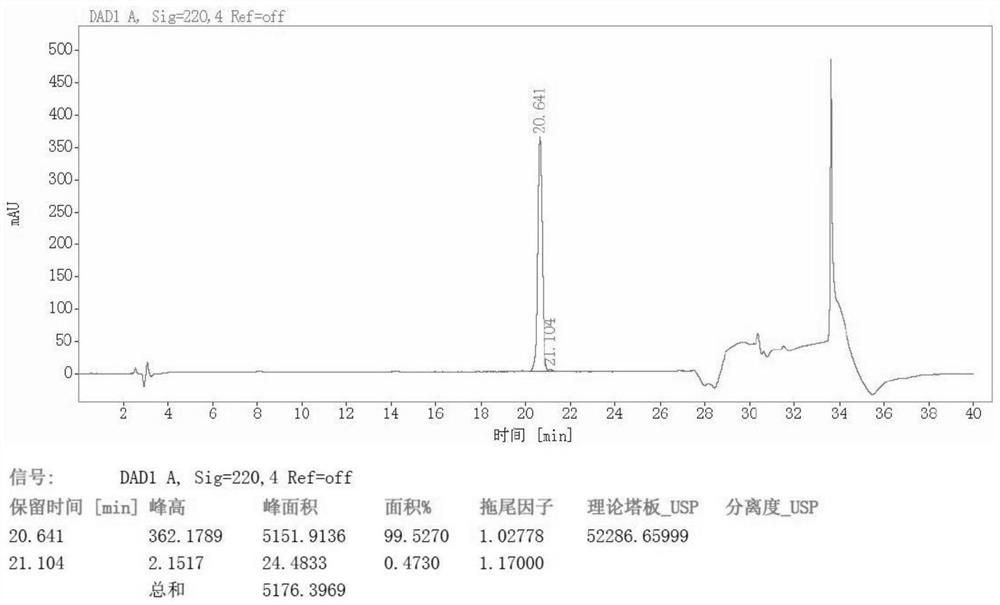
Image
Examples
preparation example Construction
[0052] The invention discloses a preparation method of teduglutide, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0053] Some abbreviations used in the present invention have the following meanings:
[0054]
[0055]
[0056] The invention provides a method for preparing teduglutide, comprising the following ...
Embodiment 1
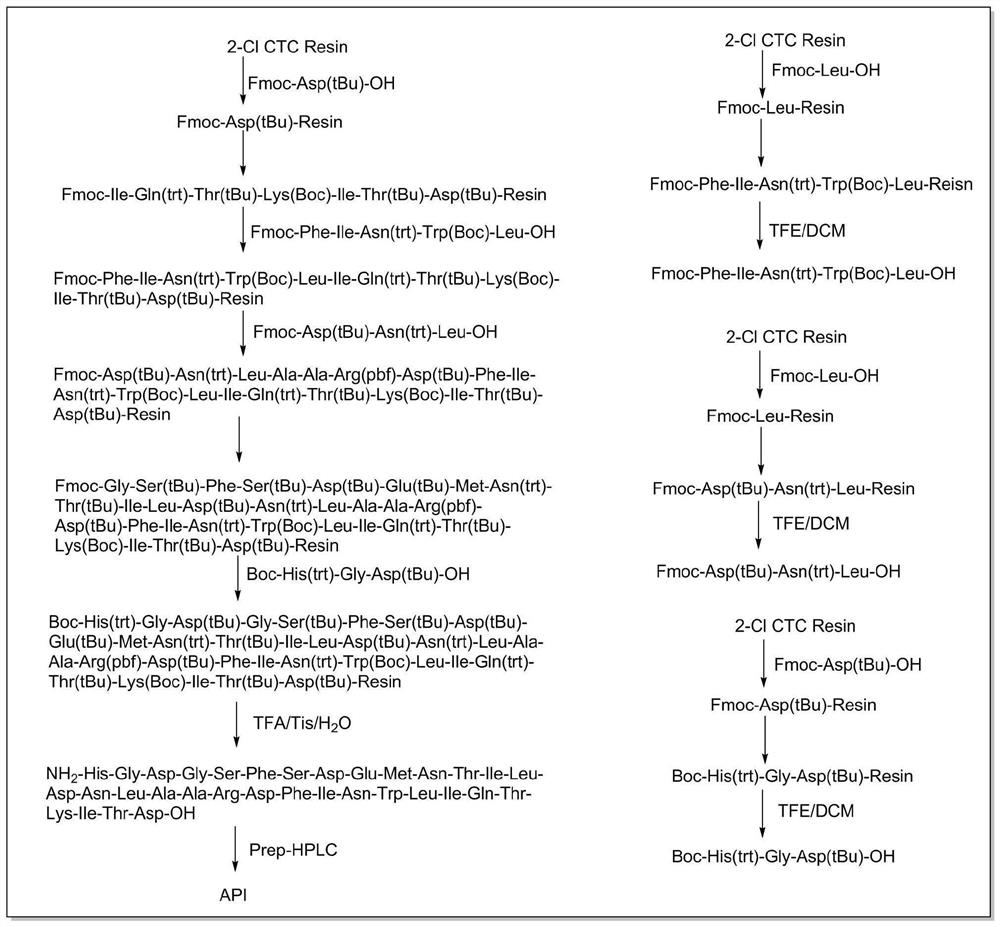
[0078] A synthetic method for teduglutide, such as figure 2 shown, including the following steps:
[0079] 1. Synthesis of the first peptide resin
[0080] Weigh 2-Cl CTC resin (1.500g, 1.5mmol) with a degree of substitution of 1.013mmol / g, add it to a solid-phase reaction vessel, add DCM (15mL) to swell for 10min, and wash the resin 3 times with DCM after swelling. 15 mL each time, the resin was vacuum-dried for later use.
[0081] Fmoc-Asp(tBu)-OH (0.191g, 0.45mmol) and DIEA (0.4mL, 2.25mmol) were dissolved in DCM (15mL), and added to the above 2-Cl CTC resin to start the reaction after the dissolution was clarified. The temperature was controlled between 25 and 30°C, and the reaction was carried out for 2.5 hours. After the reaction was completed, the resin was washed 3 times with DCM, 15 mL each time. Add methanol / DIEA / DCM=1 / 2 / 7 (volume ratio) (15mL) to the resin, and cap it at 25-30°C for 15 minutes. After the reaction, the resin is drained and the capping operation ...
Embodiment 2
[0113] A synthetic method for teduglutide, comprising the following steps:
[0114] 1. Synthesis of the first peptide resin
[0115] Weigh 2-Cl CTC resin (3.000g, 3.00mmol) with a degree of substitution of 1.013mmol / g, add it to a solid-phase reaction vessel, add DCM (30mL) to swell for 10min, and wash the resin 3 times with DCM after swelling. 30 mL each time, the resin was vacuum-dried for later use.
[0116] Fmoc-Asp(tBu)-OH (0.381g, 0.90mmol) and DIEA (0.8mL, 2.50mmol) were dissolved in DCM (30mL), and added to the above 2-Cl CTC resin to start the reaction after the dissolution was clarified. The temperature was controlled between 25 and 30°C, and the reaction was carried out for 2.5 hours. After the reaction was completed, the resin was washed 3 times with DCM, 30 mL each time. Add methanol / DIEA / DCM=1 / 2 / 7 (volume ratio) (30mL) to the resin, and cap it at 25-30°C for 15 minutes. After the reaction, the resin is drained and the capping operation is repeated once more. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com