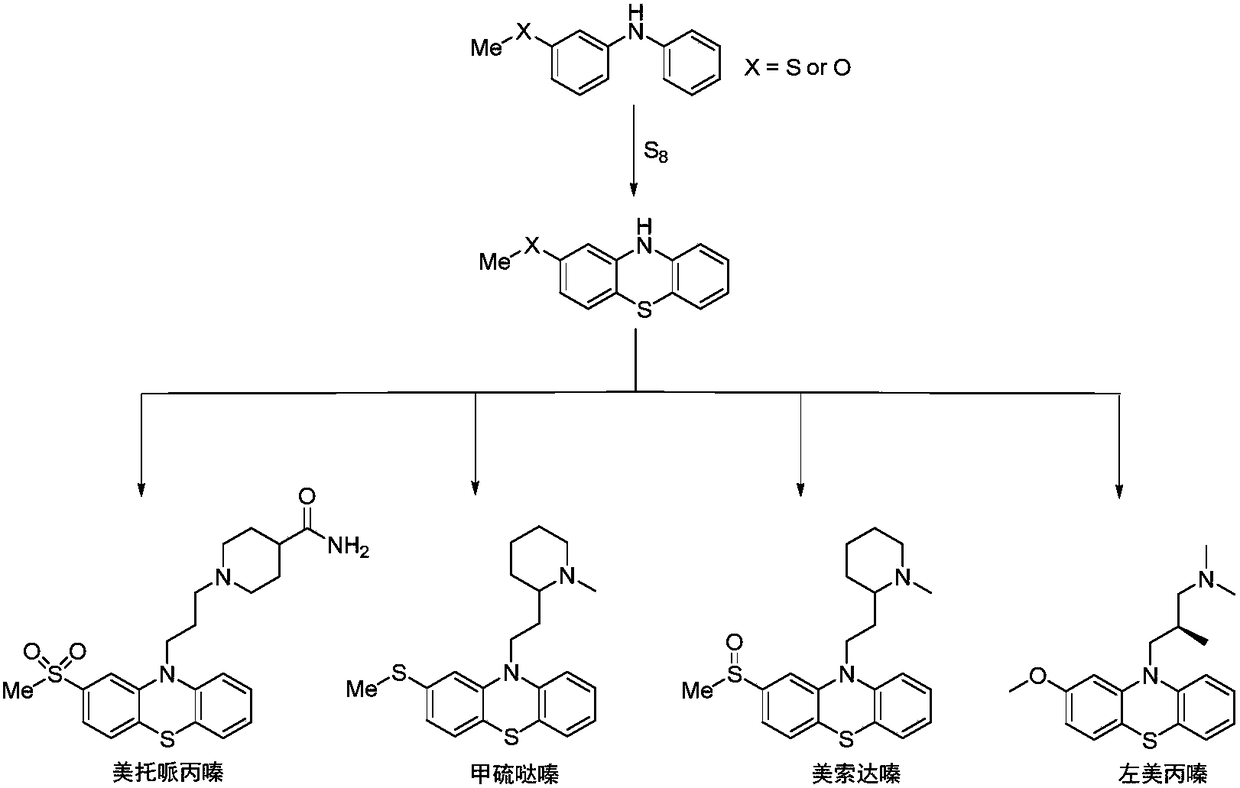
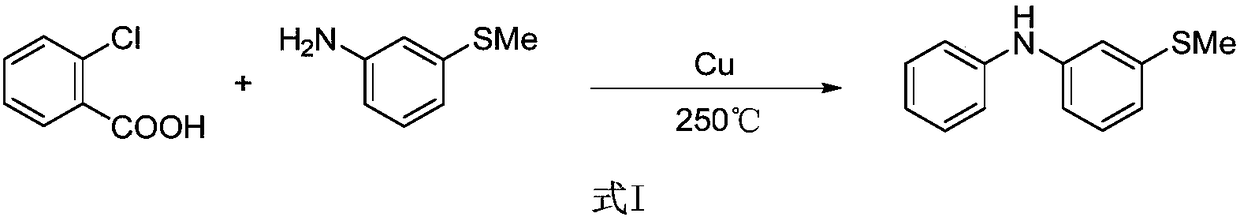
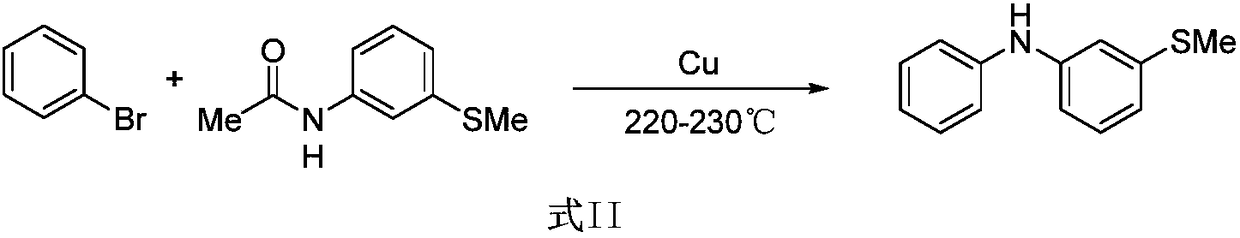
Method for synthesizing 3-(methylthio) dibenzenamine by light/nickel concerted catalysis
A technology of methylthiodiphenylamine and methylthioaniline is applied in the field of synthesis of 3-methylthiodiphenylamine, a key intermediate of pyridazine drugs, and can solve the problems of expensive catalysts and ligands, and high industrial production costs. Achieve high coupling efficiency, shorten reaction time and reduce cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Under a nitrogen atmosphere, add 3-methylthioaniline (11.62g, 83.5mmol) and bromobenzene (8.74g, 55.7mmol) into a 250mL reaction flask containing 100mL N,N-dimethylformamide, and then add NiBr 2 ·3H 2 O (0.30g, 1.114mmol), photocatalyst 1a (10.50mg, 0.011mmol), ethylene glycol dimethyl ether (0.10g, 1.114mmol), N,N-dimethylcyclohexylamine (12.76g, 100.26mmol ), the reaction solution was raised to 50°C, and reacted for 12 hours under the irradiation of blue light with a wavelength of 465nm. After the reaction, the light and heating were stopped, and the reaction bottle was cooled to room temperature. Methylformamide, N,N-dimethylcyclohexylamine; add n-hexane to dilute the residual liquid, filter to remove insoluble inorganic salts in the residual liquid, concentrate the filtrate, and distill under reduced pressure to obtain 10.7 g of 3-methylthiodiphenylamine, Yield 89%.
Embodiment 2
[0037] In this example, the photocatalyst 1a in Example 1 was replaced with an equimolar photocatalyst 1b, and other steps were the same as in Example 1 to obtain 11.1 g of 3-methylthiodiphenylamine with a yield of 92.5%.
Embodiment 3
[0039] In this example, the photocatalyst 1a in Example 1 was replaced with an equimolar photocatalyst 1c, and other steps were the same as in Example 1 to obtain 10.4 g of 3-methylthiodiphenylamine with a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com