Anti-coronavirus antibody and application thereof
A technology of antibodies and variants, applied in the direction of antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve the problems of weak neutralization activity and low target affinity, and achieve excellent neutralization activity, excellent blocking effect, high The effect of binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
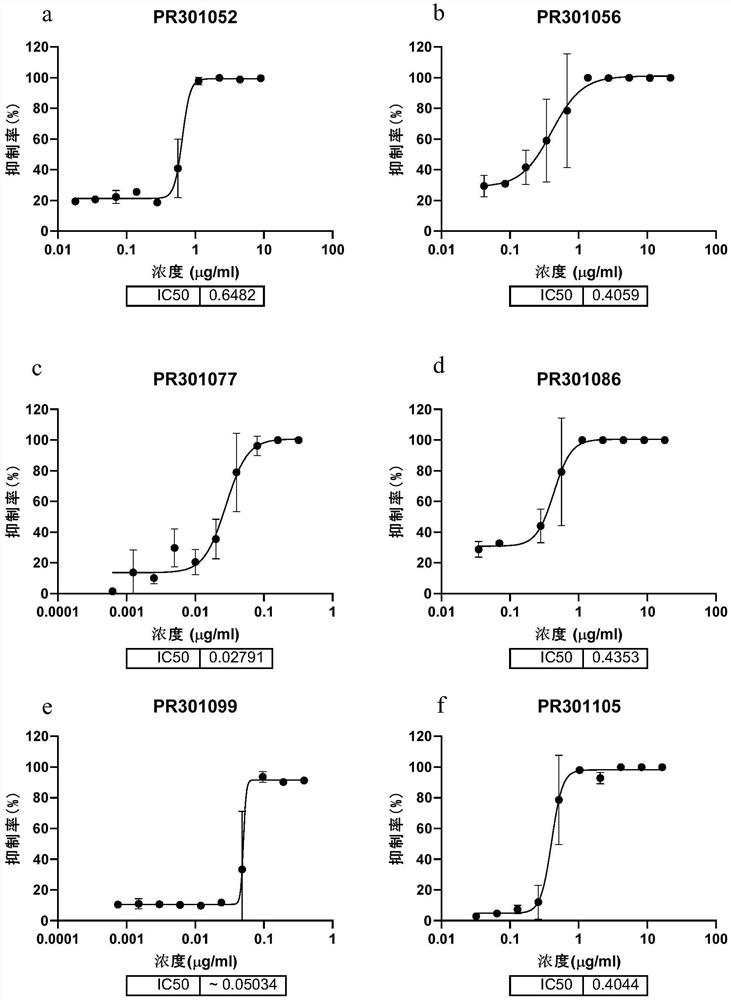
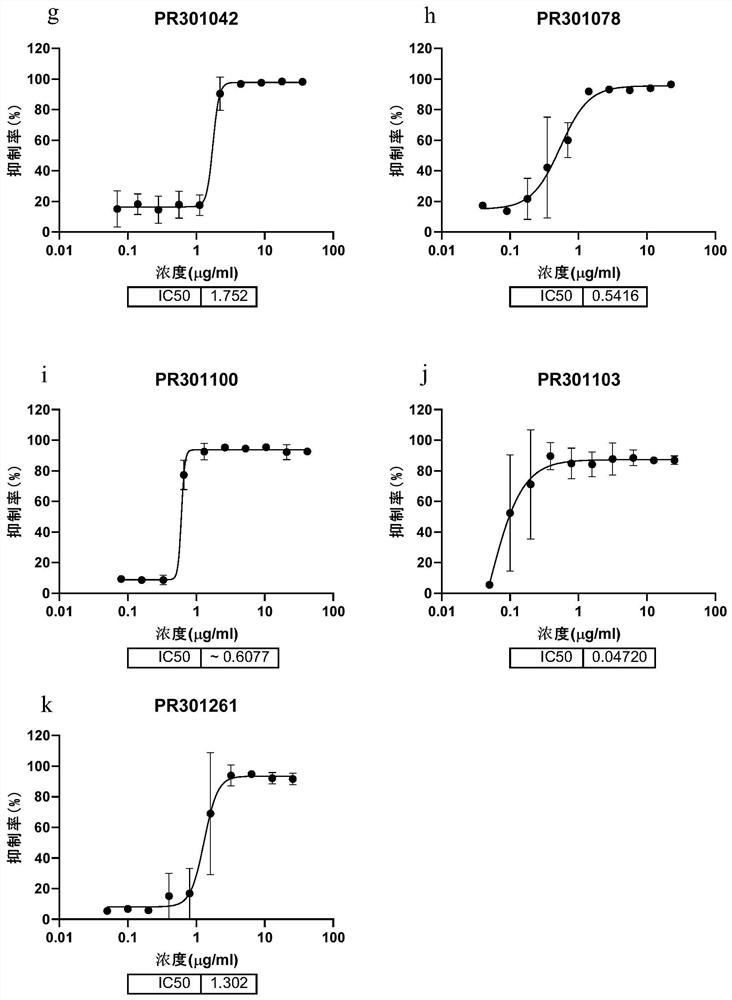
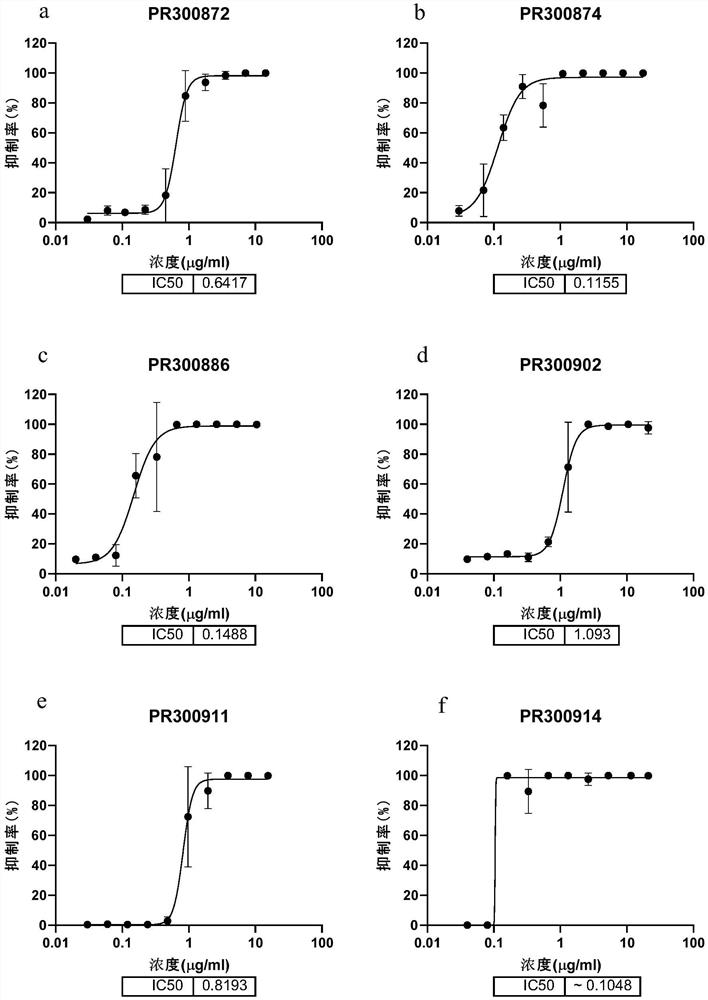
[0174] Example 1 Preliminary acquisition of candidate antibodies capable of efficiently binding to the new crown RBD protein and the new crown S protein
[0175] Use the RBD domain (receptor binding domain) in the S protein of the 2019 novel coronavirus (SARS-CoV2-2019) to immunize wild-type (BALB / c, a total of 5) or humanized (H2L2, a total of 10) Mice, and the splenocytes and bone marrow cells of the mice whose plasma reacted positively to the antigen were selected and enriched with Miltenyi (Miltenyi Biotec, #130-092-530) and Stem Cell kit (Stemcell, #18957) Antibody-expressing plasma cells (plasma B cells). Then through Beacon (Berkeley Lights, Optofluidic System) initially investigated the binding of single B cell culture supernatant to the new crown RBD protein, and derived the positive cells that the culture supernatant can effectively bind the new crown RBD, and then amplified the variable region of the antibody in a single B cell by molecular cloning sequence, and ...
Embodiment 2
[0180] Example 2 Candidate positive antibodies were transiently expressed and purified using HEK293 cells
[0181] The mammalian expression vectors of the positive antibody strains screened in Example 1 were transfected into HEK293F suspension cells (Gibco, #R79007) using PEI (SIGMA, #24885), and cultured in the culture conditions for 7 days. Culture conditions: 37°C, 5% carbon dioxide, 125rpm. After the culture supernatant was harvested, it was purified by affinity chromatography using protein A (AmMag Protein A Magnetic Beads, Genscript, L00695) purification filler. The purity of the obtained protein was preliminarily checked by SDS-PAGE (SurePAGE, Bis-Tris, 10x8, 4-12%, 12wells, Genscript, M00653).
[0182] The expression of all candidate proteins in HEK293 transient expression system was between about 10mg / L-100mg / L. And the SDS-PAGE results of these candidate antibodies all showed that the purity of the obtained antibodies was >90%.
[0183] The above transient express...
Embodiment 3
[0184] The ELISA binding activity investigation of embodiment 3 candidate positive antibody
[0185] Use the positive candidate antibody obtained in the previous step to detect its binding activity to the new crown S protein by ELISA
[0186] Method description: S protein SARS-CoV-2 (2019-nCoV) Spike Protein (S1 Subunit, His Tag), (Sino Biological, #40591-V08H) and 2019-nCoV Spike Protein (S1 Subunit, His Tag) (Genscript, Batch number: P9FE001), coat the immunoplate overnight at 4°C, add serially diluted candidate antibodies after blocking (selected concentration for serial dilution, such as 15 μg / mL starting, 10-fold serial dilution, to 0.000015 μg / mL, a total of 7 concentrations ), react at room temperature for 1 hour, and then add the secondary antibody anti-Human IgG (Fc) HRP (Jackson Immuno Research Labs, #109-035-098) to react for 1 hour. The TMB color development kit (Chaozhou Yingchuang Biotechnology Co., Ltd., TMB-S-003) was used for color development. After the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com