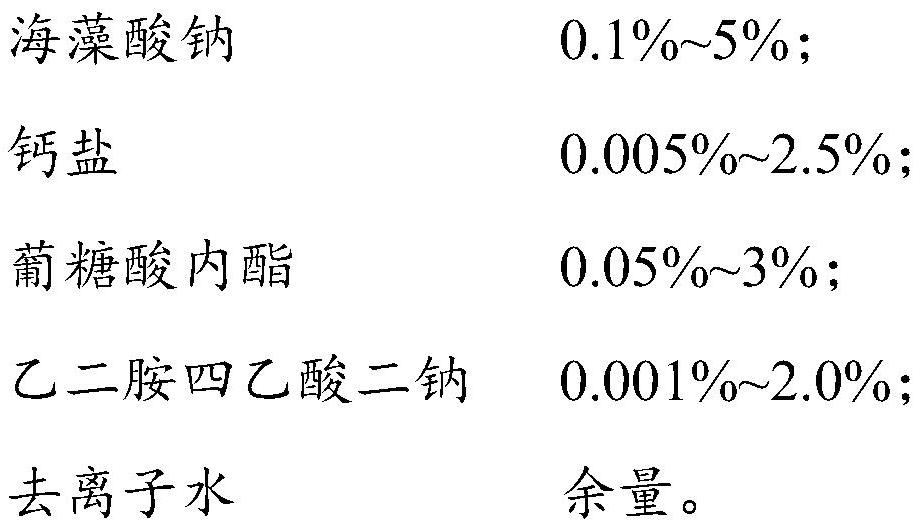
In-situ injectable anti-adhesion gel and preparation method thereof
An anti-adhesion and gel technology, which is applied in medical formula, medical science, surgery, etc., can solve the problems of difficult control of hydrogel uniformity and time-consuming, and achieve no harmful substance residue, less energy consumption, and safe raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for preparing an in-situ injectable anti-adhesion gel, specifically comprising the steps of:
[0037] 1) Weigh 97.0 g of water, then add 2.0 g of sodium alginate and 1.0 g of gluconolactone under stirring at 700 rpm, and stir for 30 min until they are completely dissolved to obtain a homogeneous solution A.
[0038]2) Weigh 0.04g of calcium sulfate and 0.03g of disodium ethylenediaminetetraacetate, add to 20.0g of water and stir to dissolve to obtain solution B.
[0039] 3) After the above solution A and solution B are prepared, they are filled with a double three-way injection device. The two needles are filled with 2mL of solution A and 1mL of solution B respectively. Anti-adhesion gel sterile product.
Embodiment 2
[0041] A method for preparing an in-situ injectable anti-adhesion gel, specifically comprising the steps of:
[0042] 1) Weigh 97.0 g of water, then add 2.0 g of sodium alginate and 1.0 g of gluconolactone under stirring at 700 rpm, and stir for 30 min until they are completely dissolved to obtain a homogeneous solution A.
[0043] 2) Weigh 0.02g of calcium sulfate and 0.015g of disodium ethylenediaminetetraacetate, add to 20.0g of water and stir to dissolve to obtain solution B.
[0044] (8) 3) After the above-mentioned solution A and solution B are prepared, they are filled with a double three-way injection device. The two needle tubes are filled with 3mL of solution A and 2mL of solution B respectively. An injectable anti-adhesion gel sterile product.
Embodiment 3
[0046] A method for preparing an in-situ injectable anti-adhesion gel, specifically comprising the steps of:
[0047] 1) Weigh 97.3g of water, then add 1.8g of sodium alginate and 0.9g of gluconolactone under stirring at 600rpm, and stir for 25min until they are completely dissolved to obtain a homogeneous solution A.
[0048] 2) Weigh 0.02g of calcium chloride and 0.015g of disodium ethylenediaminetetraacetate, add to 20.0g of water and stir to dissolve to obtain solution B.
[0049] 3) After the above-mentioned solution A and solution B are prepared, they are filled with a double three-way injection device. The two needles are filled with 3mL of solution A and 2mL of solution B respectively. Anti-adhesion gel sterile product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com