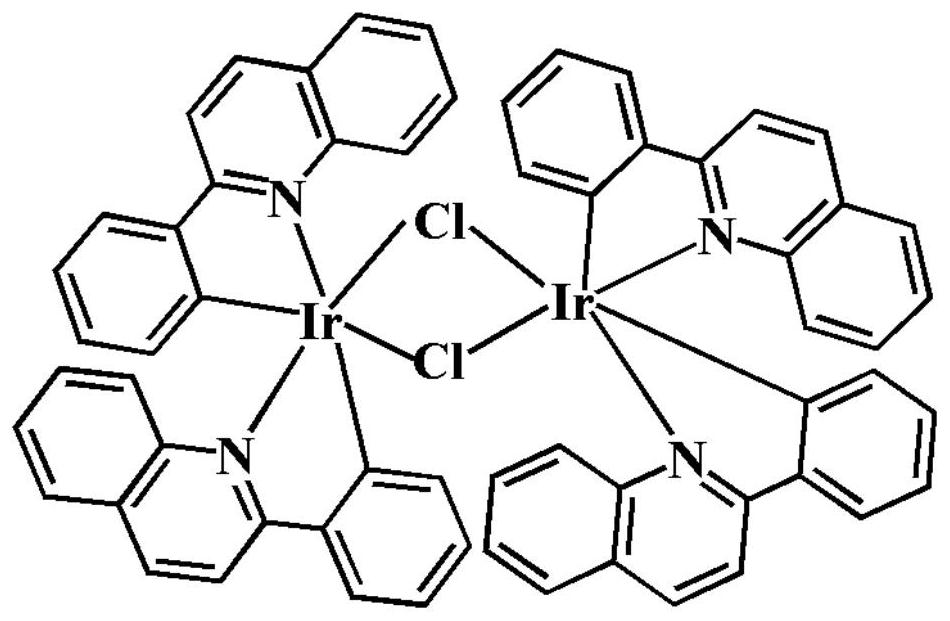
Quinoline iridium complex targeting lung cancer cis-platinum drug-resistant cells as well as synthesis method and application thereof
A technology of drug-resistant cells and synthesis method, applied in the field of medicine, can solve the problems of synthesis of metal iridium complexes that have not been reported and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a high-temperature pressure-resistant tube with a volume of 25.0 mL, add 0.1 mmol of iridium dimer [Ir(2pq) 2 Cl] 2 And 0.2mmol 4-bromo-8-hydroxyquinoline (4Br) or 5-bromo-8-hydroxyquinoline (5Br), after mixing, add dropwise 3.0mL methanol and 0.2mL dichloromethane, under sealed conditions, heat to 100°C, reacted for 48.0h, precipitated reddish-brown bulk crystals or reddish-brown powder, filtered, and dried in a vacuum oven at 55°C to obtain the target complexes 4BrIr and 5BrIr with yields of 73.5% and 82.3%.
[0034] Identification of the resulting complexes 4BrIr and 5BrIr:
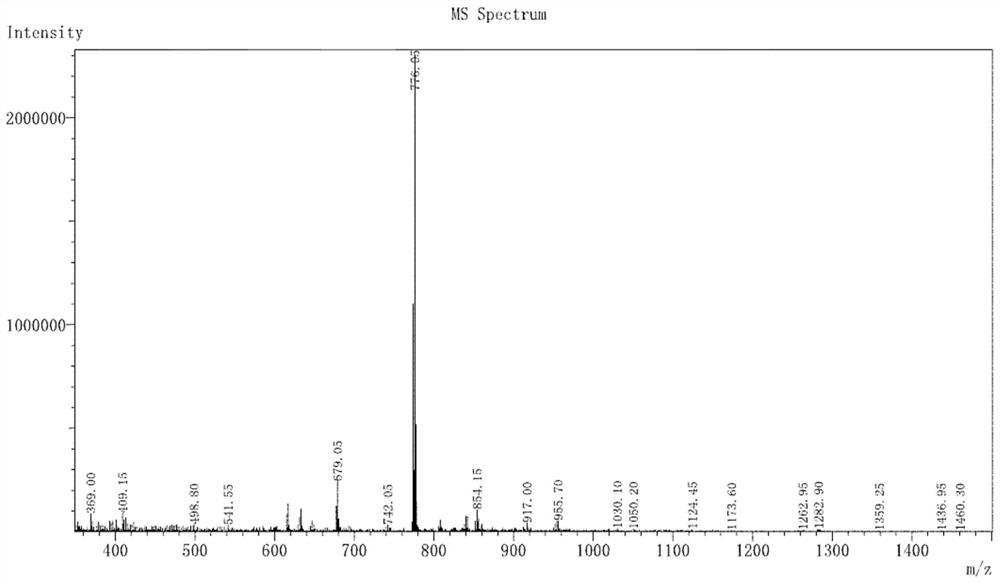
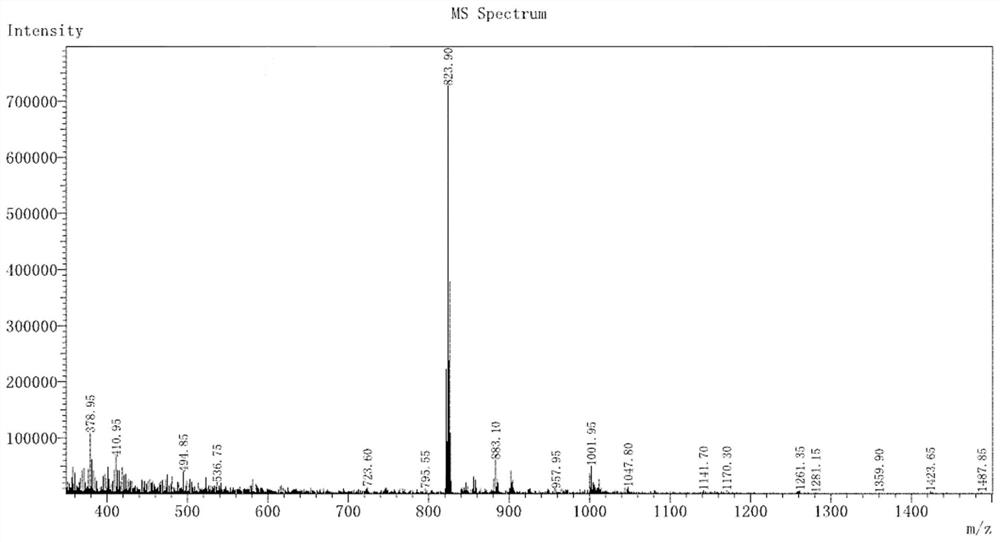
[0035] (1) The electrospray mass spectrogram of complex 4BrIr and 5BrIr, its spectrogram is as figure 2 with 3 , where M is the molecular weight of each complex.
[0036] Complex 4BrIr: ESI-MS m / z:776.05[M-(phq)+2(DMSO)] + ;
[0037] Complex 5BrIr: ESI-MS m / z:776.05[M+H] + .
[0038] (2) The single crystal structure diagram of the complex, such as Figure 4 shown.
[0039] (3) The ...
Embodiment 2
[0047] In a high-temperature pressure-resistant tube with a volume of 25.0 mL, add 0.1 mmol of iridium dimer [Ir(2pq) 2 Cl] 2 And 0.2mmol 4-bromo-8-hydroxyquinoline (4Br) or 5-bromo-8-hydroxyquinoline (5Br), after mixing, add 1.0mL methanol and 5.5mL dichloromethane dropwise, under sealed conditions, heat Reaction at 100°C for 48.0 hours, reddish-brown massive crystals or reddish-brown powder precipitated, filtered, and dried in a vacuum oven at 55°C to obtain the target complexes 4BrIr and 5BrIr with yields of 76.2% and 80.9%.
Embodiment 3
[0049] In a high-temperature pressure-resistant tube with a volume of 25.0 mL, add 0.1 mmol of iridium dimer [Ir(2pq) 2 Cl] 2 And 0.2mmol 4-bromo-8-hydroxyquinoline (4Br) or 5-bromo-8-hydroxyquinoline (5Br), after mixing, add 1.0mL methanol and 1.0mL dichloromethane dropwise, under sealed conditions, heat to 100°C, reacted for 48.0h, precipitated reddish-brown massive crystals or reddish-brown powder, filtered, and dried in a vacuum oven at 55°C to obtain the target complexes 4BrIr and 5BrIr with yields of 74.9% and 80.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com