Method for improving thermal stability of aspergillus niger xylanase through N-glycosylation modification
A technology of thermostability and xylanase, applied in the field of genetic engineering, can solve problems such as poor thermostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 xylanase mutant
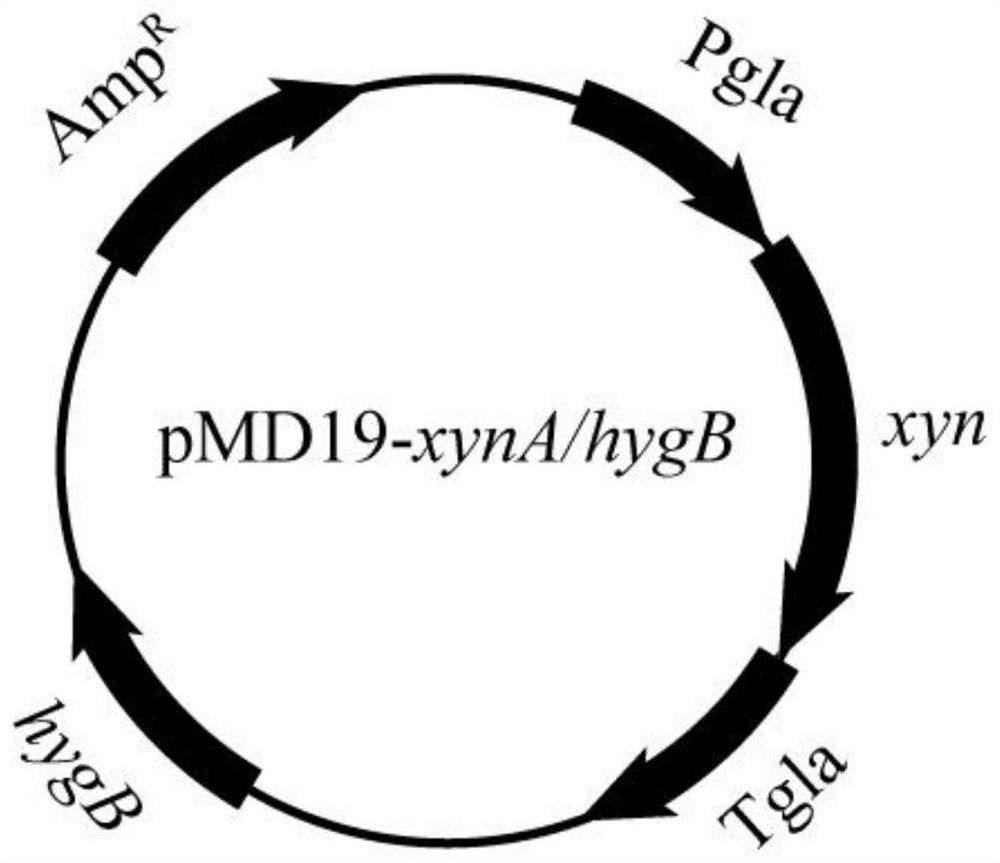
[0033] (1) Construction of recombinant Aspergillus niger A.niger / xynA / hyg
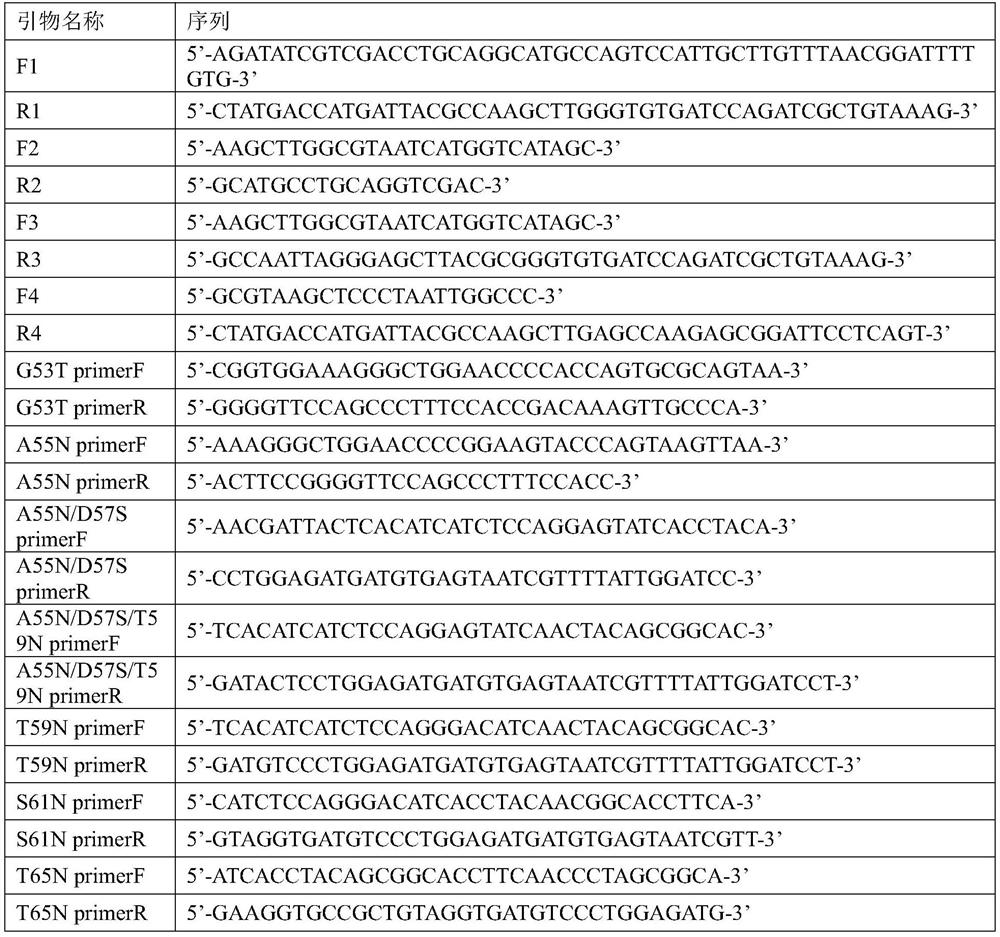
[0034] Using the genome of Aspergillus niger (A. niger) as a template, the expression cassette of xylanase xynA was amplified by PCR by designing upstream and downstream primers F1 and R1 (Table 2). Using the pMD19 vector as a template, the pMD19 vector fragment was amplified by primers F2 and R2, and the xylanase xynA expression cassette and the pMD19 vector fragment were subjected to agarose gel electrophoresis, and the products were recovered by gelation. The recovered xylanase The xynA expression cassette and the pMD19 vector fragment were cloned and ligated in one step to obtain the recombinant vector pMD19-xynA, which was verified by sequencing, and a recombinant recombinant expressing the wild-type xylanase gene xynA (nucleotide sequence shown in SEQ ID NO.1) was successfully constructed Plasmid pMD19-xynA.
[0035] After the recombinant...
Embodiment 2
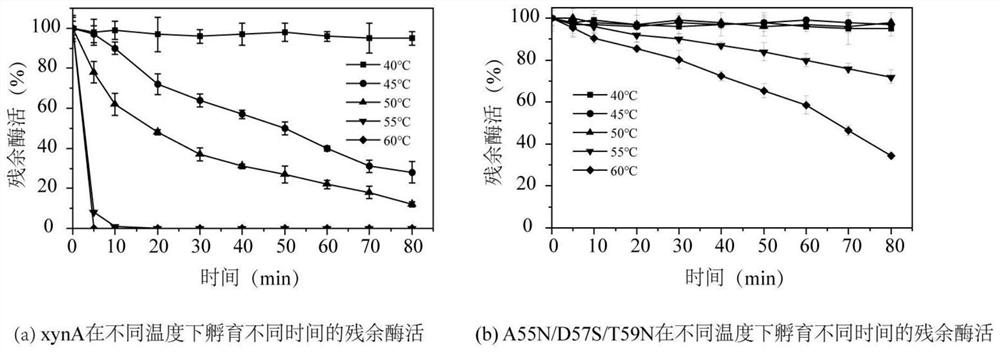
[0043] Example 2 Expression, purification and enzymatic property determination of XynA and mutants
[0044] The recombinant Aspergillus niger A.niger / xynA / hyg and A.niger / G53T / hyg, A.niger / A55N / D57S / hyg, A.niger / T59N / hyg, A.niger / S61N / hyg constructed in Example 1 , A.niger / T65N / hyg and A.niger / A55N / D57S / T59N / hyg were respectively inoculated in PDA liquid medium, and cultivated overnight at 28°C and 180rpm to obtain seed liquid, and the seed liquid was mixed with a volume ratio of 10 The amount of % was inoculated in 30mL of fresh PDA liquid medium. After culturing for 36 hours at 28°C and 180rpm, the culture solution was collected, and the culture solution was centrifuged at 12000rpm to collect the supernatant, and the supernatant was filtered through a 0.22μm filter membrane Use HisTrap TM FF purification, desalting column Sephadex G25 desalting to obtain purified wild-type enzyme xynA and mutant enzymes G53T, A55N / D57S, T59N, S61N, T65N and A55N / D57S / T59N, and determine th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com