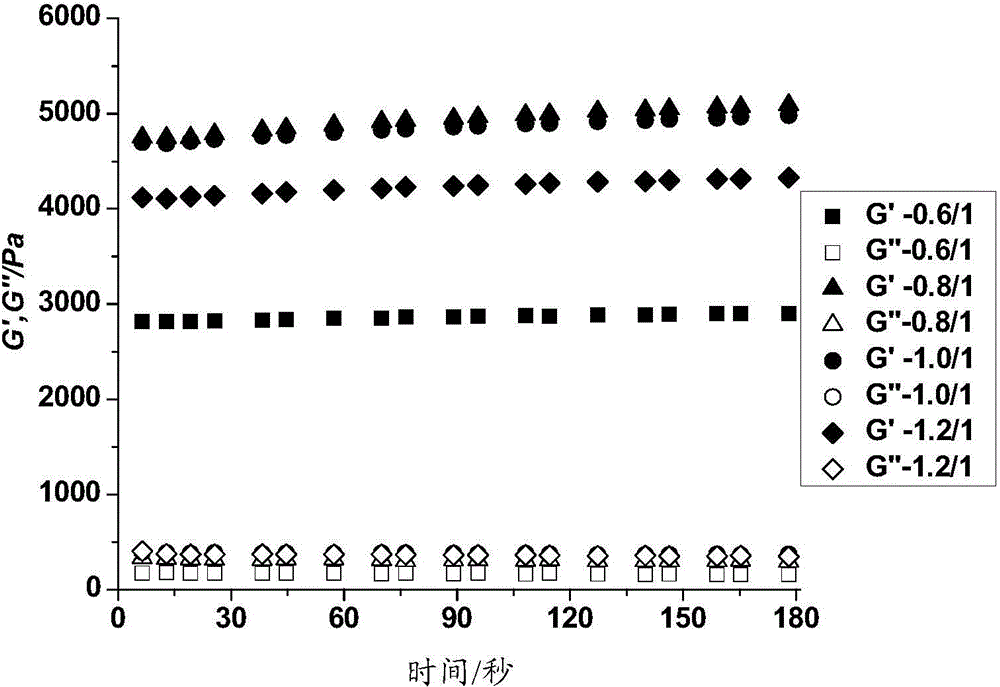
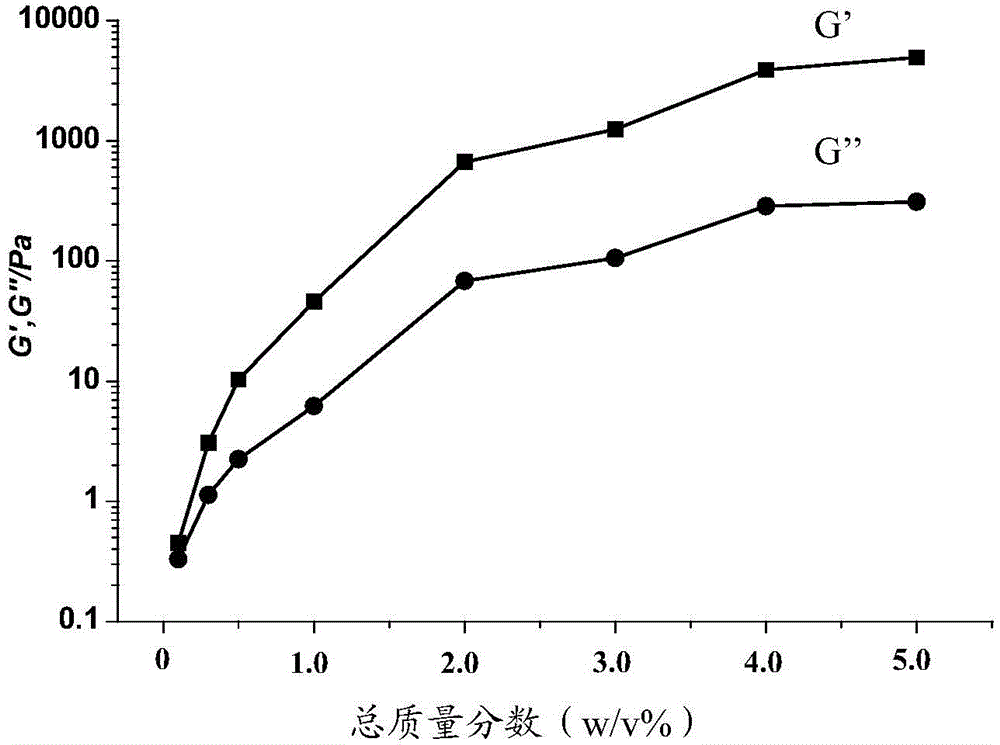
Polypeptide-DNA hydrogel and preparation method
A technology of hydrogel and polypeptide, which is applied in the field of biomedicine, can solve the problems that hydrogel needs to be deepened, and achieve the effect of good mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
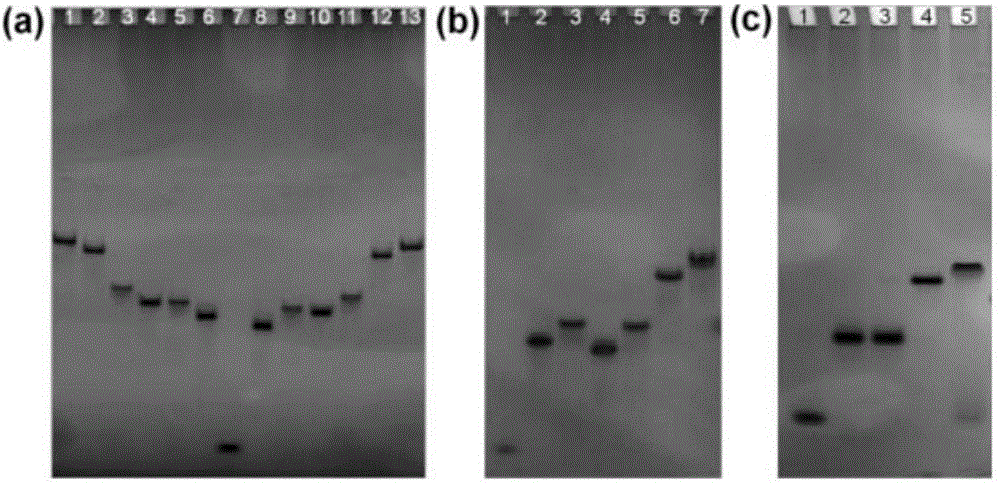
[0064] Example 1: Single-stranded DNA molecule (ssDNA) synthesis
[0065] Using the solid-phase phosphoramidite DNA synthesis method, the single-stranded DNA molecules shown in Table 1 were synthesized using an ABI394 DNA synthesizer, as follows:
[0066] Fix the solid-phase carrier containing a specific base in a DNA synthesizer, synthesize it according to the sequence shown in Table 1, and then use a concentrated ammonia solution for 3 hours at 60 degrees Celsius to ammonolyze it, remove ammonia through a vacuum concentrator, and pass the obtained crude product through a high-efficiency After purification by liquid chromatography (eluent: triethylamine acetate buffer, 100 mM, pH 7.0), the protective group containing DMT (dimethoxytrityl) was manually removed with 3% trifluoroacetic acid Then, the filter was washed with deionized water to remove DMT, and the target product was obtained. The structure of the target product was verified by MALDI-TOF-MS (Ionization Time-of-Flight ...
Embodiment 2
[0069] Example 2: Double-stranded DNA molecule (dsDNA) synthesis
[0070] According to the following steps, using the ssDNA obtained in Example 1 as a raw material, the double-stranded DNA molecules shown in Table 2 were synthesized, as follows:
[0071] Mix the stoichiometric ssDNA with 1×TBE buffer containing 100mM NaCl, then heat the resulting solution to 95 degrees Celsius, keep it warm for 3 minutes, and then cool it to room temperature within 2 hours to obtain the target double-stranded DNA molecule. Among them, 12a12b, RaRb, and HaHb have a cohesive end with a length of 12 bases, 8a8b has a cohesive end with a length of 8 bases, and 8Ma8Mb has a cohesive end with a length of 8 bases, but contains a mismatch site in its cohesive end point, RaRb and HaHb each independently have restriction endonuclease sites, respectively Ecor I site (5'-GAATTC-3') and BamH I site (5'-GGATCC-3').
[0072] Table 2
[0073] dsDNA
Embodiment 3
[0075] Embodiment 3: the preparation of polypeptide
[0076] Preparation of 5-azido-pentanoic acid
[0077] 5-Bromo-valeric acid (2.715 g, 15 mmol) was dissolved in 30 ml of dimethyl sulfoxide (DMSO) and heated to 40 °C, then sodium azide (3.25 g, 50 mmol) was gradually added, followed by heating at 85 °C , reacted overnight under stirring conditions, cooled the temperature of the obtained mixture to 40 degrees Celsius, gradually added concentrated hydrochloric acid (7mL), then stirred overnight, extracted the obtained reaction product with ether (5×80ml), collected the ether phase, It was washed successively with 10% sodium bicarbonate solution (2×100ml) and water (6×100ml), then dried over anhydrous sodium sulfate, filtered, and evaporated to dryness to obtain a yellow oily product, namely 5-azido-valeric acid.
[0078] The analytical results of the yellow oily product are as follows:
[0079] 1 H-NMR (CDCl 3 ,400MHz):δ1.63-1.76(m,4H),2.40(t,J=7.4Hz,2H),3.30(t,J=6.9Hz,2H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com