Biochemically produced sandalwood oil
A sandalwood oil, biochemical technology, applied in the directions of biochemical equipment and methods, essential oils/fragrance, biocides, etc., can solve problems such as hindering the growth of the sandalwood oil market and having little commercial use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] Preparation of sandalwood oil produced in a biochemical manner according to the present invention
[0107] The invention provides a biochemically produced sandalwood oil. Sandalwood oil is made from genetically engineered organisms. As stated above, the biochemically produced sandalwood oil of the present invention is the first sandalwood oil prepared using this technique to a commercially acceptable quality.
[0108] Sandalwood oil contains different sesquiterpene alcohol compounds that contribute to the oil's olfactory properties. Sesquiterpene alcohols are derived from terpene hydrocarbons in a multistep enzymatic process. The enzymatic synthesis of sesquiterpene alcohol compounds is known in the art. Briefly, farnesyl diphosphate (FPP) is converted to compounds such as α- and β-santalene by sesquiterpene synthase. They are subsequently converted to sesquiterpene alcohol compounds by cytochrome P450.
[0109] It will be appreciated that to prepare the biochemica...
Embodiment 1
[0276] Example 1: Preparation of sandalwood compositions of the invention in engineered E. coli cells.
[0277] Encoding N-terminal variants of Santalum album cytochrome P450 SaCP816 (WO2015040197), mint cytochrome P450 reductase (CPRm) and α-santalene / β-santalene synthase (SaSAS) (WO2010067309) Codon-optimized cDNA was designed and synthesized for optimal expression in E. coli.
[0278] The optimized cytochrome P450 and cytochrome P450 reductase cDNAs were first combined to generate a bicistronic construct, which in turn contained the P450 cDNA and a linker sequence including the ribosome binding site (RBS) and CPRm cDNA. This construct was prepared by PCR amplifying two cDNAs with 5' and 3' overhangs suitable for use with The program (Clontech) cloned at the NdeI-HindIII site of the pCWori+ expression vector (Barnes HJ (1996) Method Enzymol. 272, 3-14) provided the plasmid pCWori-SaCP816-CPRm. The codon-optimized α-santalene / β-santalene synthase cDNA was then amplified ...
Embodiment 2
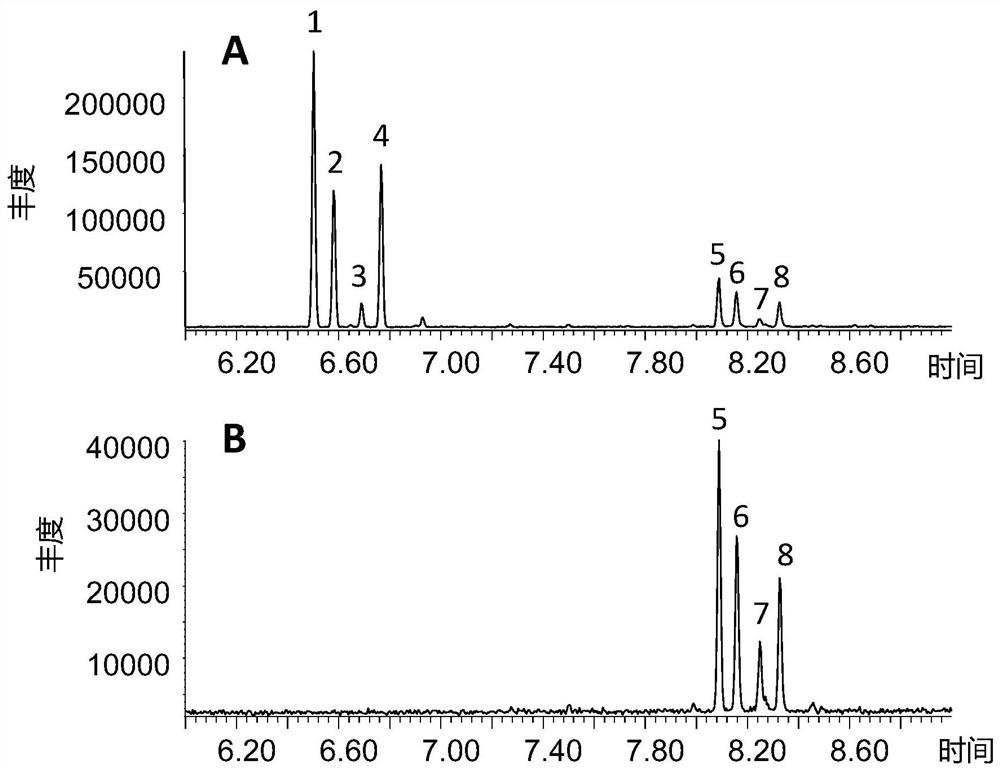
[0283] Embodiment 2: the analysis of sandalwood oil of the present invention
[0284] A kind of method of analyzing sandalwood oil composition of the present invention is provided below
[0285] Samples were diluted to 3% in dichloromethane and injected on GC / MS-FID. GC / MS-FID analysis was performed on an Agilent system equipped with two columns connected to two detectors, a mass spectrometer and an FID detector. It is equipped with two Agilent DB-1MS columns (60m x 0.25mm x 0.25μm). A 0.2 μL sample was injected in split mode (1:50) at 250°C. The initial oven temperature was held at 50°C for 5 minutes, raised to 120°C at a rate of 3°C / min, raised to 250°C at a rate of 5°C / min, held for 5 minutes, then raised to 310°C at a rate of 15°C / min, then Hold at this temperature for 20 minutes.
[0286] Mass spectra were generated at 70 eV and scanned from 0 to 20 minutes m / z: 29-250, 29-450 at 20 minutes. The linear retention index (LRI) was determined after injecting a series o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com