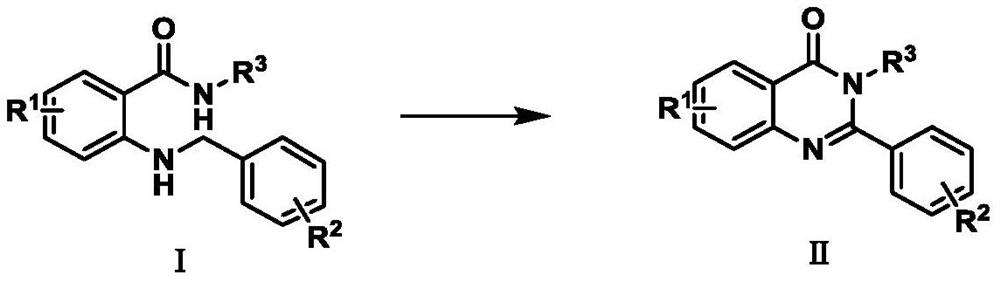
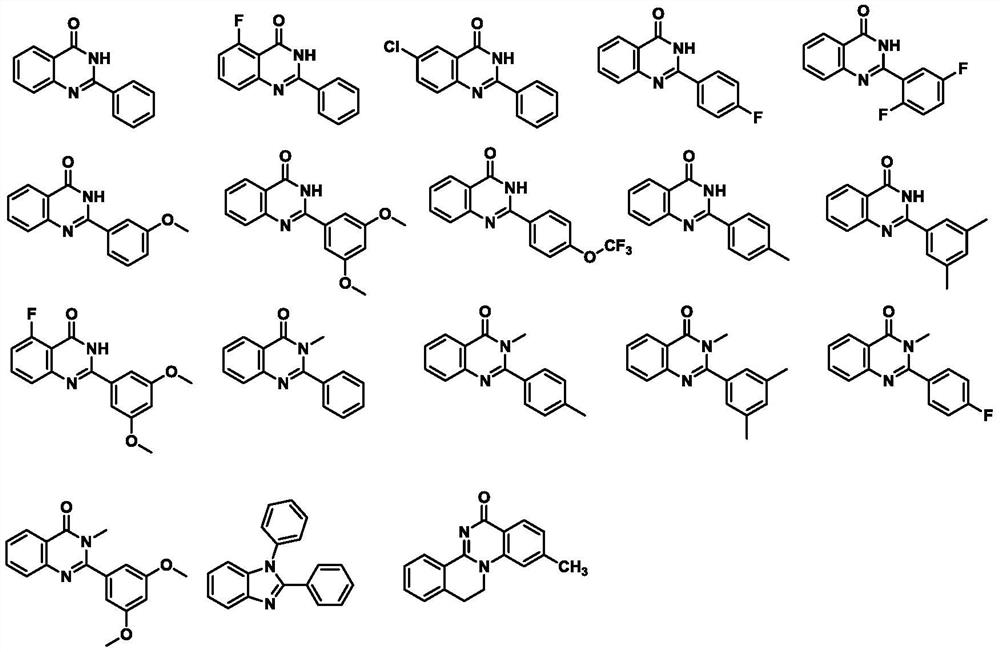
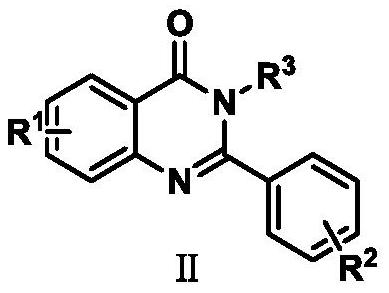
Preparation method of 2-phenyl quinazolinone compound
A technology for phenylquinazolones and compounds, which is applied in the field of 2-phenylquinazolones and their preparations, and can solve problems such as difficult separation, expensive catalysts, and poor atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the synthetic method of 2-phenylquinazolin-4 (3H)-ketone
[0020]
[0021] Add 2-(benzylamino)benzamide (0.44mmol, 100mg), elemental iodine (0.04mmol, 11mg), and dimethyl sulfoxide (2ml) into the test tube in turn, and react at 130°C for 6h. After the end, the reaction solution Extracted 3 times with ethyl acetate, the combined organic phase was concentrated to dryness, and separated by column chromatography (petroleum ether: ethyl acetate=4:1) to obtain 2-phenylquinazolin-4(3H)-one, the yield : (91 mg, 91%). 1 H NMR (400MHz, DMSO-d 6 ): δ=12.54(s, 1H), 8.21-8.15(m, 3H), 7.88-7.82(m, 1H), 7.76(d, J=7.2Hz, 1H), 7.62-7.51(m, 4H); 13 C NMR (100 MHz, DMSO-d 6 ): δ=162.71, 152.78, 149.19, 135.09, 133.16, 131.88, 129.08, 128.23, 127.97, 127.07, 126.32, 121.43.
[0022] Replace catalyst and consumption, change reaction time, reaction solvent, when reaction temperature, reaction result is as follows:
[0023]
Embodiment 2
[0024] Embodiment 2: the synthetic method of 5-fluoro-2-phenylquinazolin-4 (3H)-ketone
[0025]
[0026] Add 2-(benzylamino)-6-fluorobenzamide (0.41mmol, 100mg), elemental iodine (0.04mmol, 11mg), and dimethyl sulfoxide (2ml) into the test tube in turn, and react at 130°C for 6h. , the reaction solution was extracted 3 times with ethyl acetate, the combined organic phases were concentrated to dryness, and separated by column chromatography (petroleum ether: ethyl acetate=4:1) to obtain 5-fluoro-2-phenylquinazoline-4( 3H)-ketone, yield: (89 mg, 77%). 1 H NMR (400MHz, DMSO-d 6 ): δ=12.59(s,1H),8.20-8.17(m,2H),7.83-7.78(m,1H),7.61-7.54(m,4H),7.26-7.25(m,1H); 13 C NMR (100 MHz, DMSO-d 6 ): δ=162.29, 160.0, 159.68, 153.73, 151.32, 135.60 (d, J = 10.7Hz), 132.67, 132.13, 129.80 (d, J = 75.6Hz), 124.02, 113.33 (d, J = 20.20Hz), 110.86(d,J=6.1Hz). 19 F NMR (376MHz, DMSO-d 6 )δ=111.45.
Embodiment 3
[0027] The synthetic method of embodiment 3:6-chloro-2-phenylquinazolin-4 (3H)-ketone
[0028]
[0029] Add 2-(benzylamino)-5-chlorobenzamide (0.38mmol, 100mg), elemental iodine (0.04mmol, 11mg), and dimethylsulfoxide (2ml) into the test tube in sequence, and react at 130°C for 6h. , the reaction solution was extracted 3 times with ethyl acetate, the combined organic phases were concentrated to dryness, and separated by column chromatography (petroleum ether: ethyl acetate=4:1) to obtain 6-chloro-2-phenylquinazoline-4( 3H)-ketone, yield: (82mg, 84%). 1 H NMR (400MHz, DMSO-d 6 ): δ=12.76(s,1H),8.19(d,J=7.2,2H),8.10(d,J=2.4,1H),7.90-7.87(m,1H),7.78(d,J=8.4Hz ,1H), 7.64-7.55(m,3H); 13 C NMR (100MHz, DMSO-d 6 ): δ=161.85, 153.38, 147.91, 135.16, 132.95, 132.07, 131.22, 130.15, 129.11, 128.32, 125.35, 122.69.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com