Synthesis method of metamifop
A technology of oxazolam and a synthesis method, applied in the field of organic molecule synthesis, can solve the problems of many three wastes, high cost, low product purity and the like, and achieve the effects of high purity, reduced production cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
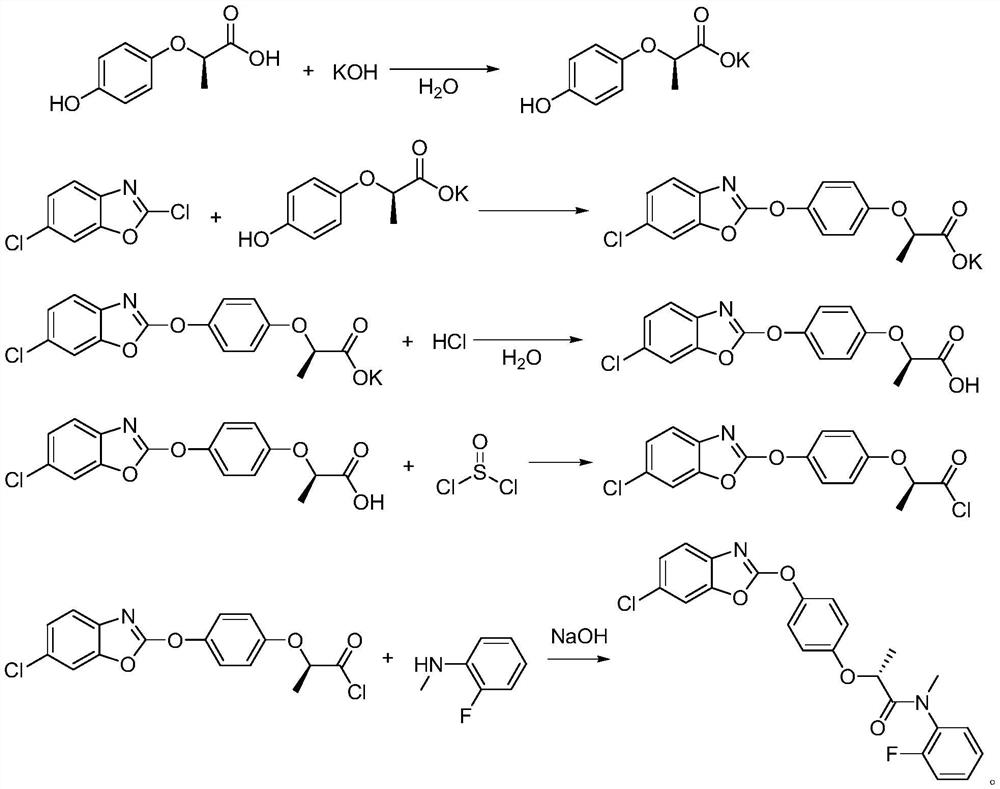
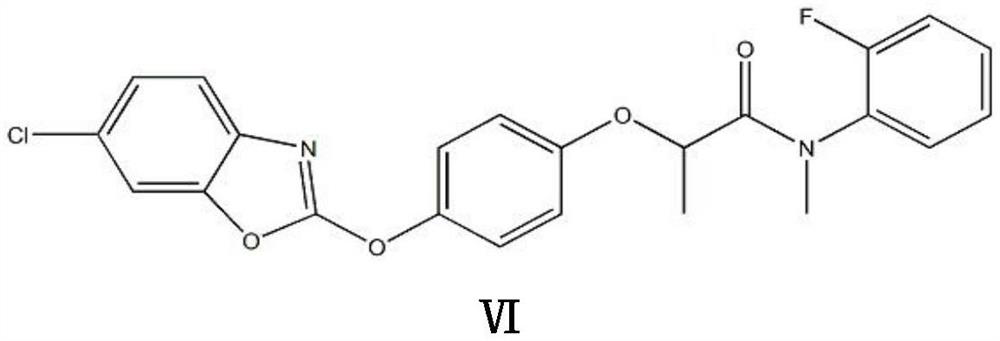
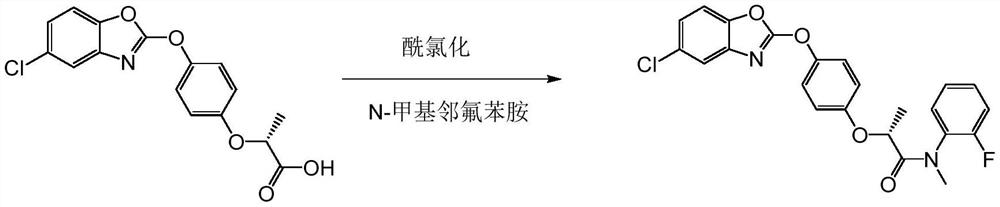
[0041] A kind of synthetic method of fenpyramid, the steps are as follows:
[0042] Step (1), in a 500ml four-necked bottle, add 57.5g of water and 76.5g of potassium hydroxide, stir and dissolve at 30°C, then put in 41.5g of R-2-(4-hydroxyphenoxy)propionic acid (Formula I) , heated up to 40°C, and kept at normal pressure for 2 hours to generate R-2-(4-hydroxyphenoxy)propionic acid potassium salt (formula II), and the conversion of R-2-(4-hydroxyphenoxy)propionic acid The rate is 100%; then add dropwise a mixture of 48 g of 2,6-dichlorobenzoxazole and 57.5 g of acetone in a four-necked bottle, and react under normal pressure at 50 to 53°C for 2 hours, R-2-(4-hydroxybenzene The conversion rate of oxy)propionate potassium salt is 98%, and the acetone is removed under negative pressure, and then suction filtered to obtain 81g of filter cake, which is 2-(4-((6-chlorobenzo[d]oxazole-2- Base) oxy) phenoxy) propionic acid potassium salt (formula III), yield 96%.
[0043]
[0044...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com