SERS biosensor and application thereof in preparation of detection system for detecting myocardial infarction miRNA
A biosensor and system technology, applied in the field of bioanalysis and detection, can solve the problem of low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
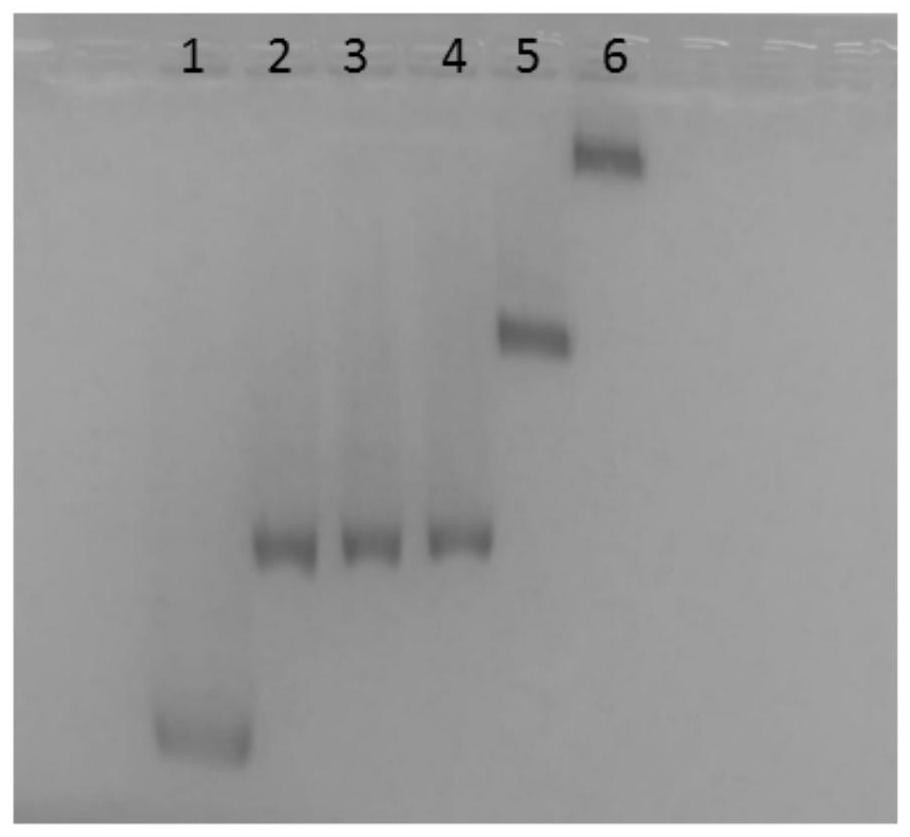
[0054] The hybrid chain amplification strategy was confirmed on a 1.5% agarose gel. Firstly, the two hairpin rings S2-HP1 and S2-HP2 were individually modified on AuNPs of about 16nm, and then, different concentrations of S1-HP2 (customized, the same as the product chain after enzyme digestion) and 10mM MgAc 2 The mixture was incubated at 37°C for 3 hours to form a hybrid chain amplification reaction. Electrophoresis was performed at 8 V / cm 3 in 0.5×TBE buffer. The final electrophoresis results are captured by a smartphone, such as figure 2 .
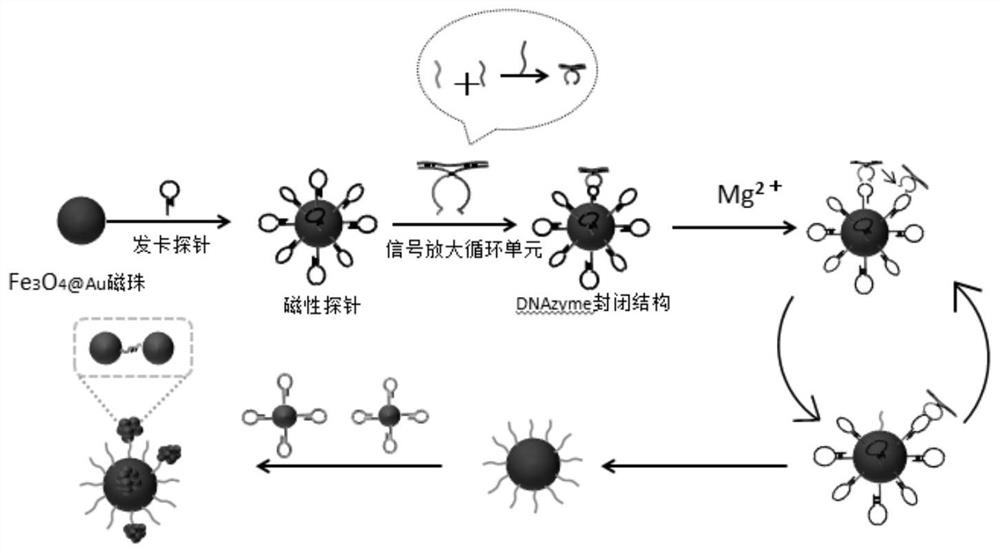
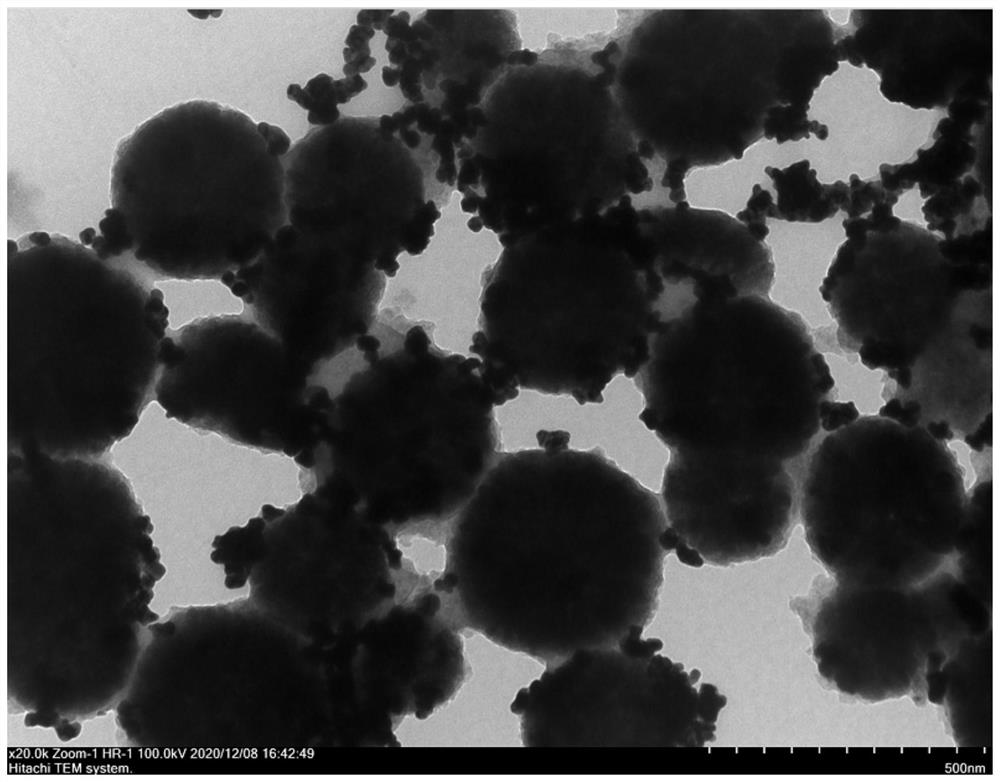
[0055] SERS sensor preparation: first take 500μL, 0.2mg / mL Fe 3 o 4 , add EDC and NHS and shake overnight (10mMEDC 5μL, 1mM NHS 5μL), the Fe 3 o 4 activation. Then the hairpin probe S1-HP and the above-mentioned activated Fe 3 o 4 Add it to 1 mL of PBS buffer with a pH of 7.4, incubate with shaking at room temperature for more than 10 h, and use H with the aid of a magnet. 2 O was washed once and redissolved in PBS buffer. T...
Embodiment 2
[0061] In Example 2 on the basis of Example 1, the detection target miRNA-499 was replaced with the detection target miRNA-328, and the steps and methods were the same as in Example 1. Such as Figure 6is the relationship graph and linear relationship between the concentration of miRNA-328 and Raman intensity, adding a series of miRNA-328 with a concentration ranging from 1fM to 1nM can significantly enhance the Raman intensity, which makes the relationship between Raman intensity and miRNA-328 concentration There is a linear relationship between the numbers. The effect is the same as miRNA-499.
Embodiment 3
[0063] In Example 3 on the basis of Example 1, the detection target miRNA-499 is replaced by the detection target miRNA-208, such as Figure 7 is the relationship graph and linear relationship between the concentration of miRNA-208 and Raman intensity, adding a series of miRNA-208 with a concentration ranging from 1fM to 1nM can significantly enhance the Raman intensity, which makes the relationship between Raman intensity and miRNA-208 concentration There is a linear relationship between the numbers. The effect is the same as miRNA-499.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com