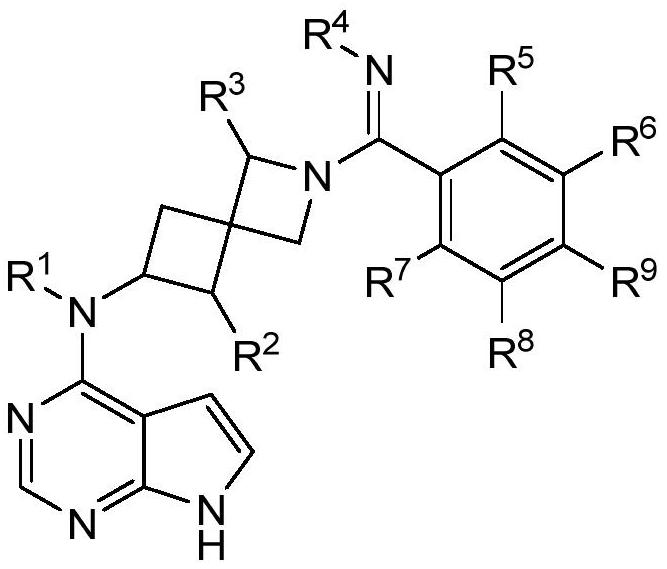
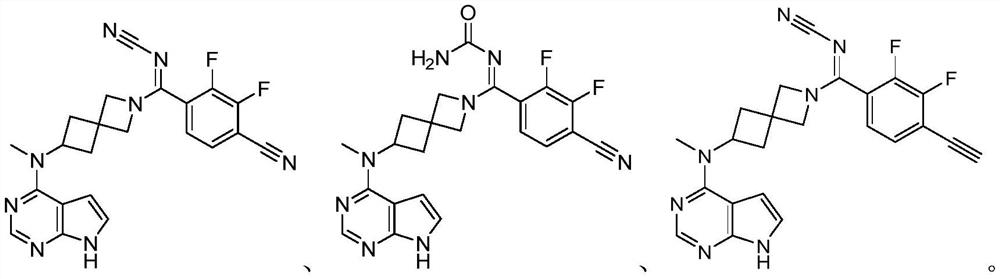
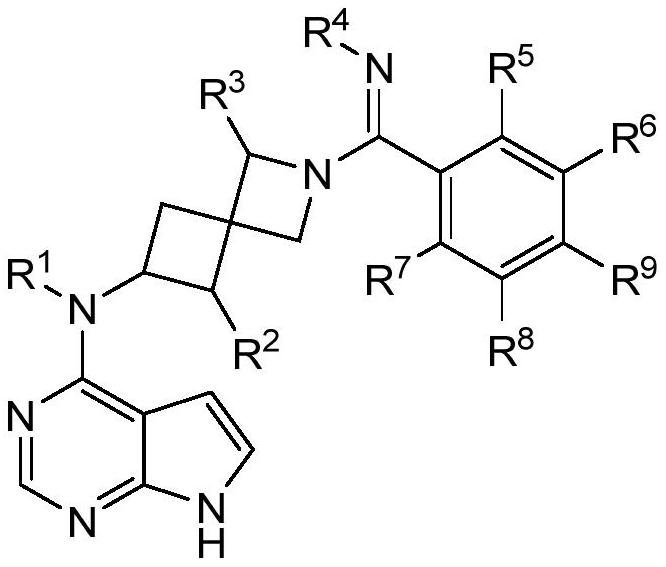
Selective JAK1 inhibitor compound as well as preparation method and application thereof
A compound, hydrate technology, applied in non-central analgesics, anti-inflammatory agents, digestive system, etc., can solve problems such as unmet clinical needs, adverse reactions, and unsatisfactory treatment effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]
[0065]
[0066] first step
[0067] To a 10 mL methanol solution of 1a (1.00 g, 4.73 mmol) was added 30% methylamine methanol solution (1.00 g, 9.66 mmol), and reacted at room temperature for 3 hours. Sodium borohydride (361 mg, 9.54 mmol) was added in portions, and the resulting reaction solution was reacted at room temperature overnight. After the reaction was completed, quenched with water (20mL), extracted with ethyl acetate (30mL x 2), combined the organic phases, washed successively with water (20mL x 1) and saturated sodium chloride solution (20mL x 1), dried over anhydrous sodium sulfate, After filtration, the filtrate was concentrated under reduced pressure to obtain a residue, and the residue was concentrated under reduced pressure to obtain crude product 1b (1.00 g), yield: 93%.
[0068] 1 H NMR (400MHz, CDCl 3 )δ3.91(s,2H),3.83(s,2H),3.10-3.06(m,1H),2.46-2.41(m,2H),2.32(s,3H),1.87-1.82(m,2H) ,1.43(s,9H).
[0069] second step
[0070] 1b (1.00g,...
Embodiment 2
[0089]
[0090]
[0091] first step
[0092] 1 (50 mg, 0.12 mmol) in 5 mL of hydrogen chloride 1,4-dioxane solution (4M) was reacted at 30° C. overnight. The reaction solution was directly purified by preparative HPLC to obtain 2 (12 mg), yield: 23%.
[0093] 1 H NMR (400MHz, DMSO-d 6 )δ11.62(s,1H),8.17-7.98(m,1H),7.91-7.72(m,1H),7.45-7.25(m,1H),7.13(s,1H),6.57(s,1H) ,6.47(s,1H),6.11(s,1H),5.25-4.96(m,1H),4.23-3.75(m,4H),3.20(dd,J=6.9,3.4Hz,3H),2.60-2.51 (m,4H).
[0094] MS-ESI calculated value [M+1] + 451, the measured value is 451.
Embodiment 3
[0096]
[0097] first step
[0098] To a solution of 3a (1.00 g, 4.22 mmol) and potassium carbonate (637 mg, 4.61 mmol) in 10 mL of N,N-dimethylformamide was added iodomethane (659 mg, 4.64 mmol), and the resulting reaction solution was reacted overnight at room temperature. After the reaction was completed, it was diluted with water (20 mL), extracted with ethyl acetate (20 mL x 1), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain 3b (486 mg), which was directly used in the next reaction.
[0099] 1 H NMR (400MHz, CDCl 3 )δ7.69-7.63(m,1H),7.46-7.41(m,1H),3.99(s,3H).
[0100] second step
[0101] Under nitrogen protection, 3b (386mg, 1.54mmol), ethynyltrimethylsilane (226mg, 2.30mmol), bistriphenylphosphine palladium dichloride (21mg, 0.03mmol) and cuprous iodide (3mg, 0.15 mmol) in 10 mL of triethylamine was reacted overnight at 80°C. After the reaction was completed, cooled, filtered, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com