Synthesis method of phenylhydrazone derivative of aloe pines
A synthetic method, the technology of aloe vera pine, applied in the field of biomedicine, achieves the effect of short reaction time, simple operation steps and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
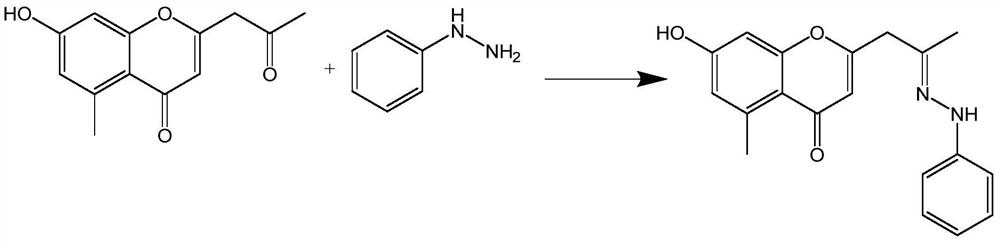
[0025] The synthetic method of the phenylhydrazone derivative of embodiment 1 aloe vera pine
[0026] (1) Compound 1-1 (10mmoL, 2.322g) was dissolved in 20mL butanol, then phenylhydrazine (10mmoL, 1.081g) and auxiliary agent (10mmoL, 0.845g) were added, and the reaction was stirred at room temperature 25°C for 3h, Concentrate under reduced pressure to obtain a concentrate; the auxiliary agent is p-toluenesulfonic acid and sodium acetate (molar ratio 0.3:10);
[0027] (2) The concentrate was separated and purified by column chromatography (petroleum ether: ethyl acetate = 4:1, v / v) to obtain a yellow oily product (2.163 g, yield 67%).
Embodiment 2
[0028] The synthetic method of the phenylhydrazone derivative of embodiment 2 aloe pine
[0029] (1) Dissolve compound 1-1 (10mmoL, 2.322g) in 20mL butanol, then add phenylhydrazine (12mmoL, 1.296g) and additives (12mmoL, 1.015g), and stir the reaction at room temperature 25°C for 3h, Concentrate under reduced pressure to obtain a concentrate; the auxiliary agent is p-toluenesulfonic acid and sodium acetate (molar ratio 0.3:10);
[0030] (2) The concentrate was separated and purified by column chromatography (petroleum ether: ethyl acetate = 4:1, v / v) to obtain a yellow oily product (2.226 g, yield 69%).
Embodiment 3
[0031] The synthetic method of the phenylhydrazone derivative of embodiment 3 aloe vera pine
[0032] (1) Compound 1-1 (10mmoL, 2.322g) was dissolved in 20mL butanol, then phenylhydrazine (12mmoL, 1.296g) and auxiliary agent (12mmoL, 1.036g) were added, and the reaction was stirred at room temperature 25°C for 3h, Concentrate under reduced pressure to obtain a concentrate; the auxiliary agent is p-toluenesulfonic acid and sodium acetate (molar ratio 0.5:10);
[0033] (2) The concentrate was separated and purified by column chromatography (petroleum ether: ethyl acetate = 4:1, v / v) to obtain a yellow oily product (2.160 g, yield 67%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com