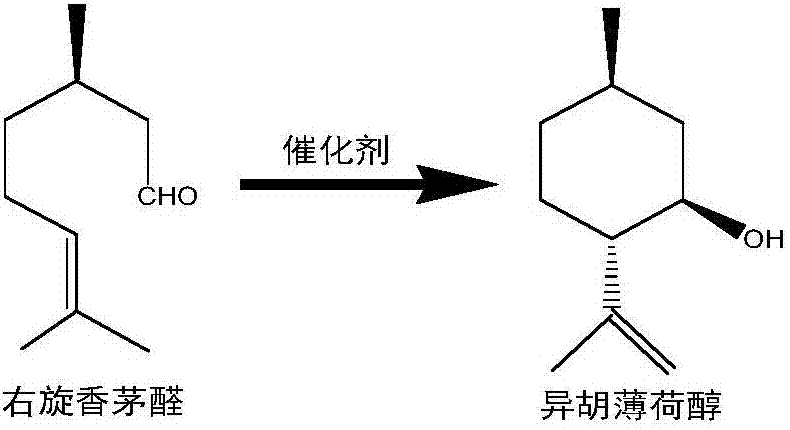
Method of preparing isopulegol by cyclization reaction of citronellal
A technology of isopulegol and cyclization reaction, which is applied in the fields of chemical instruments and methods, reduction preparation of oxygen-containing functional groups, organic chemistry, etc., can solve the problems of unsuitable large-scale industrial production, frequent catalyst recycling operations, and slow reaction rate etc. to achieve the effects of fast reaction and mass transfer rate, easy serialization and automation, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Weigh 1g of SiO with a diameter of 60-80nm 2 The particles were dispersed in 8 ml of toluene, and 0.1 g of propyltrimethoxysilane was added. Under the protection of nitrogen, it was stirred and refluxed at 110°C for 4 hours. After cooling, after high-speed centrifugation, toluene washing, and vacuum drying, the interface active nano-SiO can be obtained. 2 .
[0033] 2) 0.15g interface active nano-SiO 2 Disperse in 60ml toluene by ultrasonic wave, add 3ml 0.01mol / L phosphotungstic acid solution, stir to form Pickering emulsion. After milking, 0.6g of HSi(OCH 3 ) 3 Add it into the emulsion system, mix evenly, hydrolyze at 30°C for 24 hours, then let it stand, remove the upper oil phase, wash it with toluene three times, and you can get the microcapsules encapsulating the phosphotungstic acid catalyst.
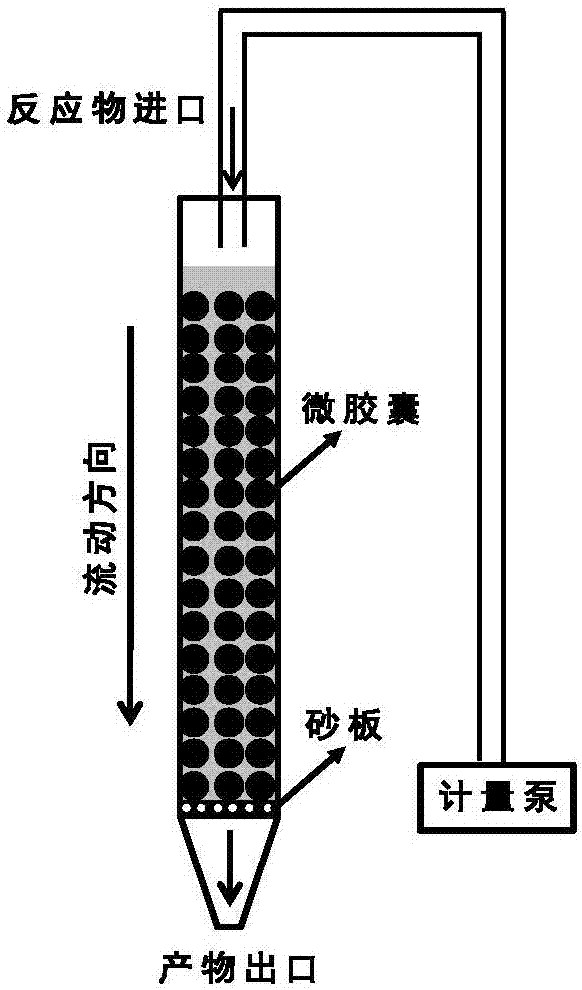
[0034] 3) Slowly transfer the microcapsules of the encapsulated phosphotungstic acid catalyst prepared above to an inner diameter of 0.8cm continuous flow reactio...
Embodiment 2
[0036] 1) Weigh 1g of SiO with a diameter of 60-80nm 2 The particles were dispersed in 8 ml of toluene, and 0.6 g of propyltrimethoxysilane was added. Under the protection of nitrogen, it was stirred and refluxed at 110°C for 4 hours. After cooling, after high-speed centrifugation, toluene washing, and vacuum drying, the interface active nano-SiO can be obtained. 2 .
[0037] 2) 0.3g interface active nano-SiO 2 Disperse in 110ml of n-octane by ultrasonic wave, add 7.5ml of phosphotungstic acid solution with a concentration of 0.05mol / L, and stir to form a Pickering emulsion. After milking, 1.5g of Si(OC 2 h 5 ) 4 Add it into the emulsion system, mix well, hydrolyze at 50°C for 24 hours, then let it stand still, remove the upper oil phase, wash it with n-octane three times, and you can get the microcapsules encapsulating the phosphotungstic acid catalyst.
[0038] 3) Slowly transfer the microcapsules of the encapsulated phosphotungstic acid catalyst prepared above to a c...
Embodiment 3
[0040] 1) Weigh 1g of SiO with a diameter of 60-80nm 2 The particles were dispersed in 8 ml of toluene, and 0.3 g of propyltrimethoxysilane was added. Under nitrogen protection, stir and reflux at 110°C for 5 hours. After cooling, after high-speed centrifugation, toluene washing, and vacuum drying, the interface active nano-SiO can be obtained. 2 .
[0041] 2) 0.5g interface active nano-SiO 2 Disperse in 150ml of n-hexane by ultrasonic waves, then add 15ml of phosphotungstic acid solution with a concentration of 0.08mol / L, and stir to form a Pickering emulsion. After milking, 1.25g of Si(OCH 3 ) 4 Add it into the emulsion system, mix evenly, hydrolyze at 30°C for 24 hours, let it stand still, remove the upper oil phase, wash it with n-hexane three times, and you can get the microcapsules encapsulating the phosphotungstic acid catalyst.
[0042] 3) Slowly transfer the microcapsules of the encapsulated phosphotungstic acid catalyst prepared above to an inner diameter of 2c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com