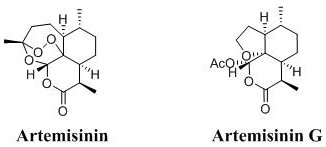
Method for preparing Artemisin G
A technology for artemisinin and raw materials, applied in the field of preparing ArtemisininG, can solve the problems of unsuitability for industrial production, cumbersome processing steps, large dosage, etc., and achieves the effects of easy handling, concise synthesis route, and simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
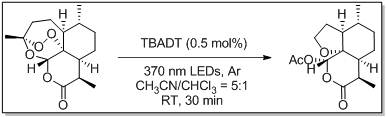
[0019] Mix 3 mL of acetonitrile and 0.6 mL of chloroform, add artemisinin (0.282 g, 1 mmol) and TBADT (13.6 mg, 0.005 mmol) at room temperature. The reaction system was an argon atmosphere, and it was placed at room temperature and reacted under 370 nm light for half an hour. The solvent was then removed to obtain a crude product, which was purified by silica gel column chromatography (PE / EtOAc = 4:1, volume ratio) to obtain a white solid (0.259 g, reaction yield 92%).
[0020] Physical state: white powdery solid;
[0021] Melting point: 91-93℃;
[0022] 1 H NMR (400 MHz, CDCl 3 ) δ 6.65 (1H, s), 4.21 (1H, t, J = 7.6 Hz), 3.94(1H, dd, J = 7.4, 15.8 Hz), 3.16 (1H, dq, J = 2.7, 7.2 Hz), 2.16 (3H, s), 1.8-2.1 (4H, m), 1.73 (1H, m), 1.60 (1H, m), 1,47 (1H, m), 1.21 (3H, d, J =7.3 Hz), 1.08 (1H, m), 0.99 (3H, d, J = 6.3 Hz).
[0023] 13 C NMR (90 MHz, CDCl 3 ) δ 12.4, 20.3, 21.1, 24.2, 27.6, 30.8, 34.6, 34.9, 46.6, 54.8, 69.1, 79.3, 92.9, 168.3, 171.5. ...
Embodiment 2
[0026] Mix 15 mL of acetonitrile and 3 mL of chloroform, add artemisinin (1.41 g, 5 mmol) and TBADT (68 mg, 0.025 mmol) at room temperature. The reaction system was filled with argon, and placed at room temperature under 370 nm light for half an hour to react. The solvent was then removed to obtain a crude product, which was purified by silica gel column chromatography (PE / EtOAc = 4:1, volume ratio) to obtain a white solid (1.33 g, 94%).
Embodiment 3
[0028] Mix 150 mL of acetonitrile and 30 mL of chloroform, add artemisinin (14.1 g, 50 mmol) and TBADT (680 mg, 0.25 mmol) at room temperature. The reaction system was filled with argon, and placed at room temperature under 370 nm light for half an hour to react. The solvent was then removed to obtain a crude product, which was purified by silica gel column chromatography (PE / EtOAc = 4:1, volume ratio) to obtain a white solid (12.8 g, 91%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com