Oxynitride hydride, metal carrier containing oxynitride hydride, and catalyst for ammonia synthesis
A technology of oxygen-nitrogen hydrides and loads, which is applied in the field of catalysts for ammonia synthesis, can solve the problems of undisclosed catalysts, and achieve the effect of excellent productivity and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0084] (Perovskite Oxygen Nitrogen Hydride)
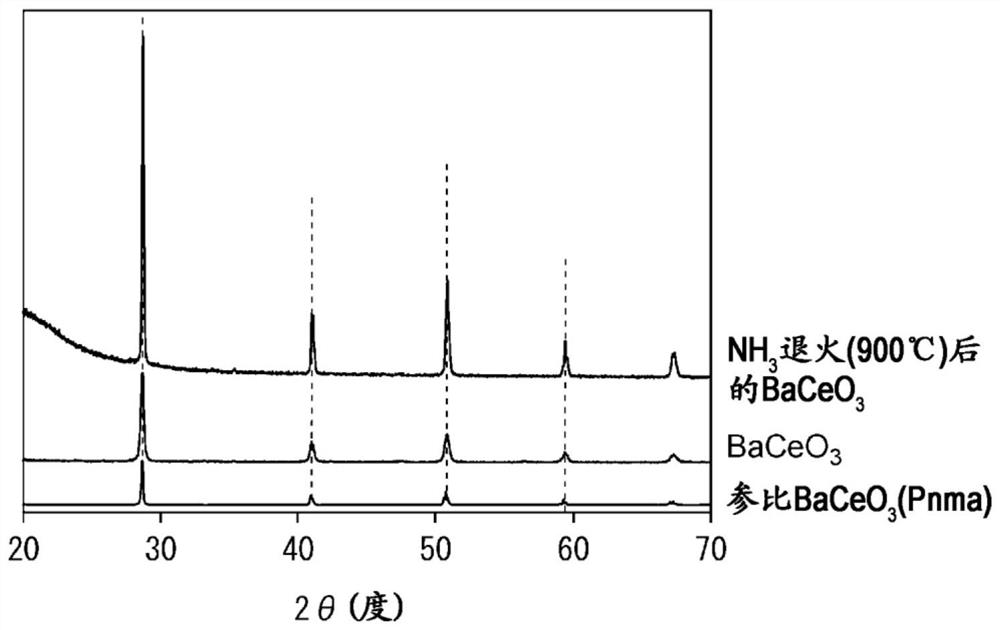
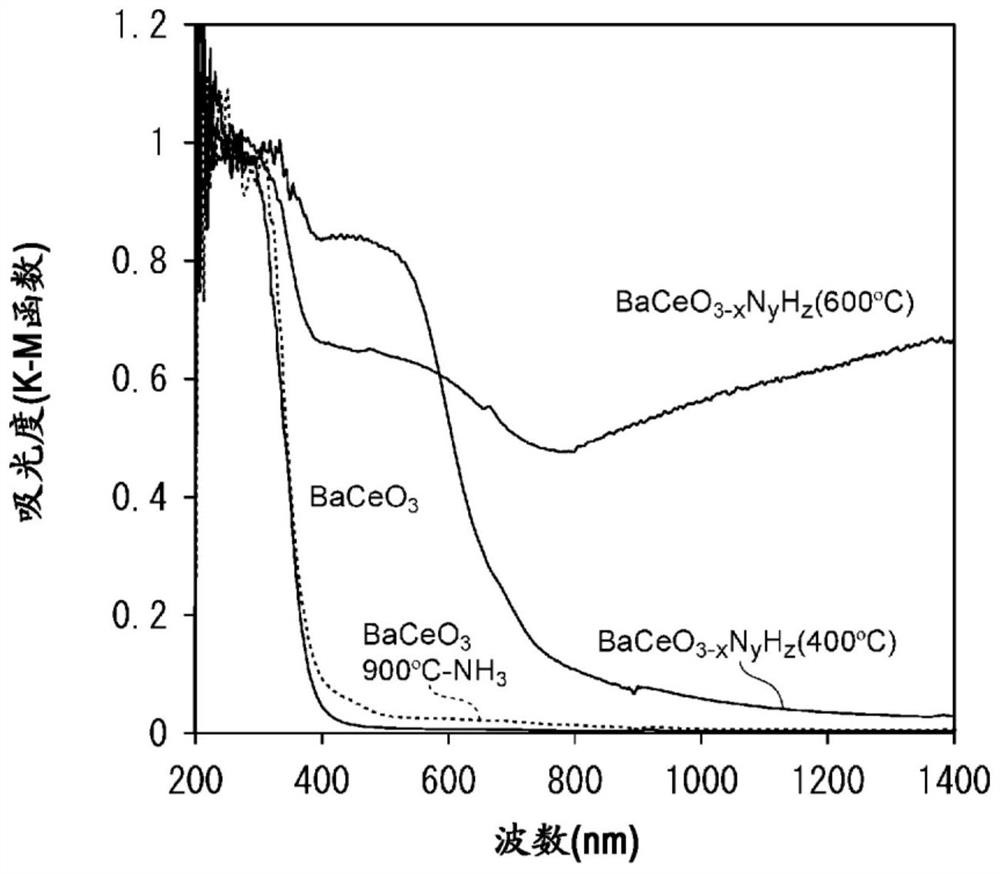
[0085] The oxynitrogen hydride of the first embodiment of the present invention is a perovskite-type oxynitrogen hydride having a perovskite-type crystal structure. Perovskite-type oxynitride hydride is a mixture of nitrogen and hydrogen in BaCeO 3 Perovskite-type oxynitride hydride obtained by doping on the oxygen site. The perovskite-type oxynitrogen hydride of the first embodiment of the present invention (hereinafter, sometimes referred to as the present embodiment) is a compound represented by the following general formula (2) and has a perovskite-type crystal structure. It has BaCeO with no nitrogen and hydrogen doping 3same type of crystal structure. That is, for the perovskite-type oxynitrogen hydride of the present embodiment, preferably, after doping BaCeO with nitrogen and hydrogen 3 While maintaining the perovskite crystal structure.
[0086] BaCeO 3-x N y h z (2)
[0087] In the above general formula (2), x r...
Embodiment 1
[0195] (Preparation of catalyst for ammonia synthesis)
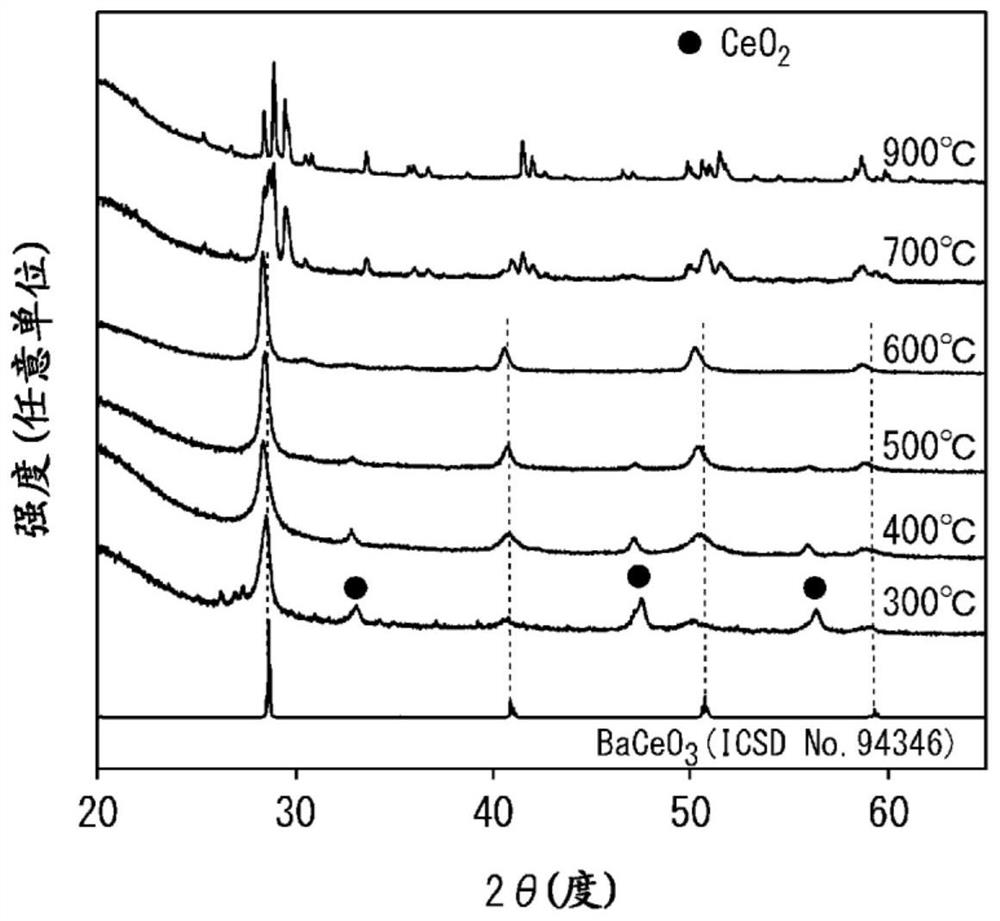
[0196] [BaCeO 3-x N y h z Synthesis of powder]
[0197] By adding CeO 2 Vacuum heat treatment was carried out at 600°C to remove water adsorbed on the surface, and the dehydrated CeO 2 and Ba(NH 2 ) 2 Mixing was performed in an Ar glove box using an agate mortar. At this time, mixing was performed so that the molar ratio of Ce and Ba became 1:1. By dissolving the obtained powder in NH 3 Heat treatment at 600 °C for 6 hours in air flow to obtain BaCeO 3-x N y h z powder.
[0198] [to BaCeO 3-x N y h z Loading of Ru]
[0199] The powdered BaCeO obtained by the above-mentioned method 3-x N y h z 0.50g and Ru 3 (CO) 12 (Aldrich, 99%) 0.056g (with respect to BaCeO 3-x N y h z In other words, as the supported metal Ru (corresponding to 5% by mass) was inserted into a quartz glass tube, heated at 70° C. for 1 hour in a vacuum, followed by heating at 120° C. for 1 hour, so that Ru 3 (CO) 12 attached to...
Embodiment 2
[0208] [to BaCeO 3-x N y h z load of Co]
[0209] The powdered BaCeO obtained by the above-mentioned method 3-x N y h z 95mg with Co 2 (CO) 8 14.5mg (relative to BaCeO 3-x N y h z In other words, Co as the supported metal (corresponding to 5% by mass) was placed in a quartz glass reaction tube, and then 15 mL / min of nitrogen gas and 45 mL / min of hydrogen gas (60 mL / min in total) were circulated in the reaction tube, and the temperature was raised for 2 hours. Up to 400 ° C, maintained for 5 hours, obtained in BaCeO 3-x N y h z The load with Co immobilized on it (hereinafter, Co / BaCeO 3-x N y h z ).
[0210] Hereinafter, ammonia synthesis was performed using the catalyst for ammonia synthesis described above.
[0211] [BaCeO loaded with Co 3-x N y h z Ammonia Synthesis]
[0212]
[0213] In addition to using the above Co / BaCeO 3-x N y h z As a catalyst to replace the Ru / BaCeO of Example 1 3-x N y h z Except, by using the same method and conditions...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com