Solid preparation of palbociclib isethionate and preparation method thereof
A technology of piperidine isethionate and piperidine isethionate, applied in the field of pharmaceutical preparations, can solve the problems of poor solubility, large hygroscopicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
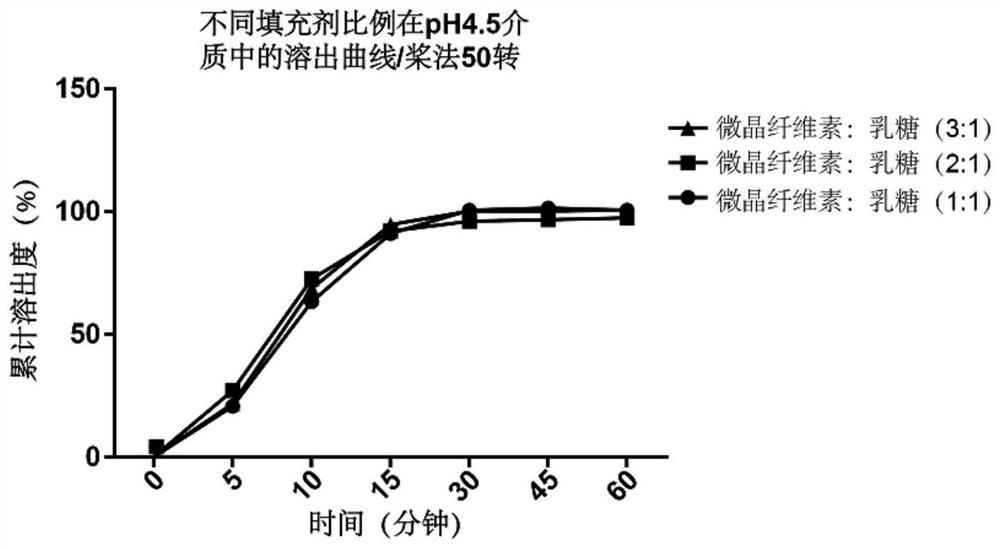
[0044] In order to investigate the ratio of filler microcrystalline cellulin to lactose, the following three formulations were prepared, and pH4.5 acetate buffer was selected as the medium for formulation screening, and the investigation index was the dissolution rate in 15 minutes in the pH4.5 medium (dissolution assay is carried out to tablet according to Pharmacopoeia), see table 2 below for details.
[0045] Table 2 The composition of prescriptions with different filler ratios
[0046]
[0047]
[0048] The results of filler ratio investigation are as follows:
[0049] From figure 1 The dissolution data is available, and the change in the ratio of the filler microcrystalline cellulose to lactose has no obvious effect on the dissolution. According to experience, the dosage of microcrystalline cellulose: lactose = 2:1 is selected.
Embodiment 2
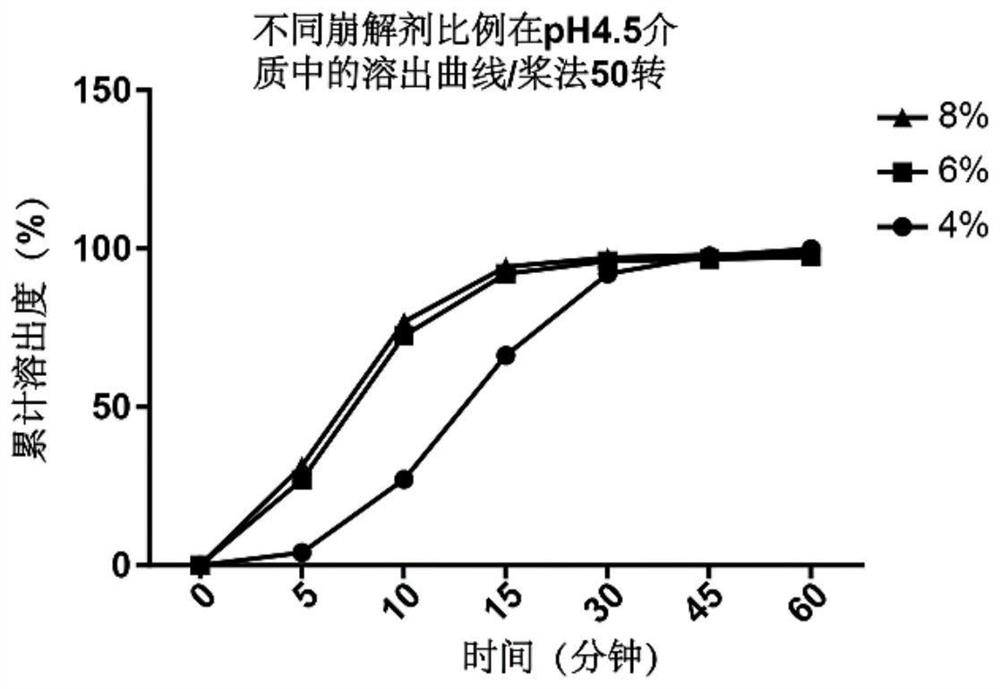
[0051] The prescriptions of 4%, 6%, and 8% disintegrants were prepared, and the dissolution rate of different proportions of disintegrants in pH4.5 medium was investigated for 15 minutes, as shown in Table 3 below.
[0052] Table 3 Preparation of different disintegrant dosage prescriptions
[0053]
[0054] The investigation result of disintegrant dosage is as follows:
[0055] From figure 2 Dissolution data are available, 4% disintegrant prescription can not reach 85% in 15 minutes, and there is no significant difference in dissolution between 6% and 8% disintegrants, so 6% disintegrant is selected as the optimal ratio .
Embodiment 3
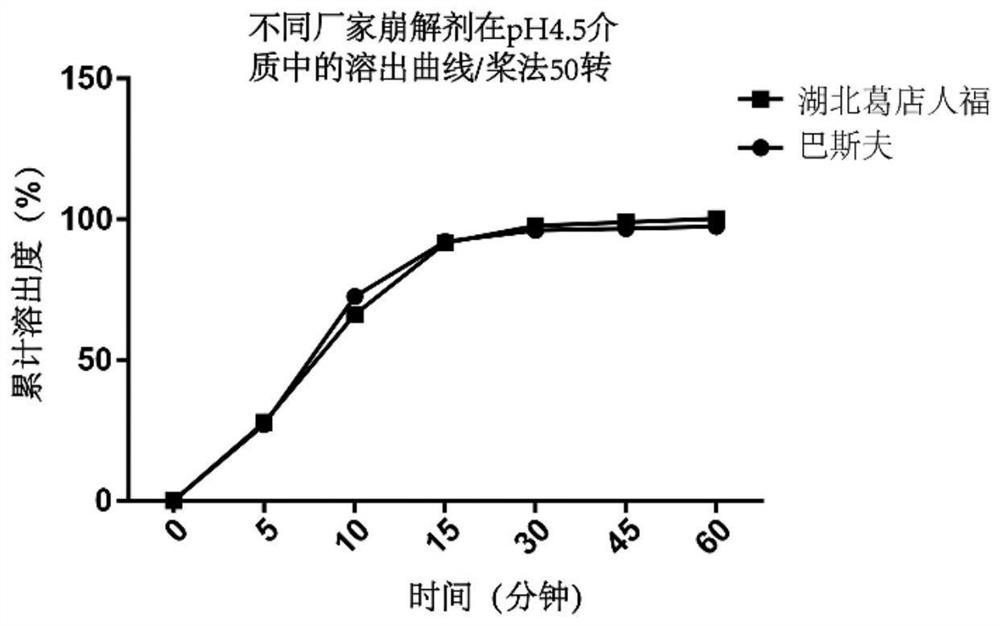
[0057] Disintegrant crospovidone is the key functional excipient in the prescription. We consider selecting disintegrants from different manufacturers for screening, preparing prescriptions, and investigating their dissolution behavior in acetate buffer at pH 4.5. See Table 4.
[0058] Table 4 Preparation of disintegrant prescriptions from different manufacturers
[0059]
[0060] The inspection results of different manufacturers of disintegrants are as follows:
[0061] From image 3 Dissolution data are available, and there is no significant difference in the dissolution of disintegrants from the two manufacturers. In this study, imported cross-linked polyvinyl chloride from BASF in Germany was selected as the disintegrant in the prescription.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com