Pharmaceutical composition for treating type 2 long QT syndrome and application thereof
A technology of QT syndrome and composition, applied in the field of medicine, can solve problems such as complex pathogenesis, and achieve the effect of increasing current density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
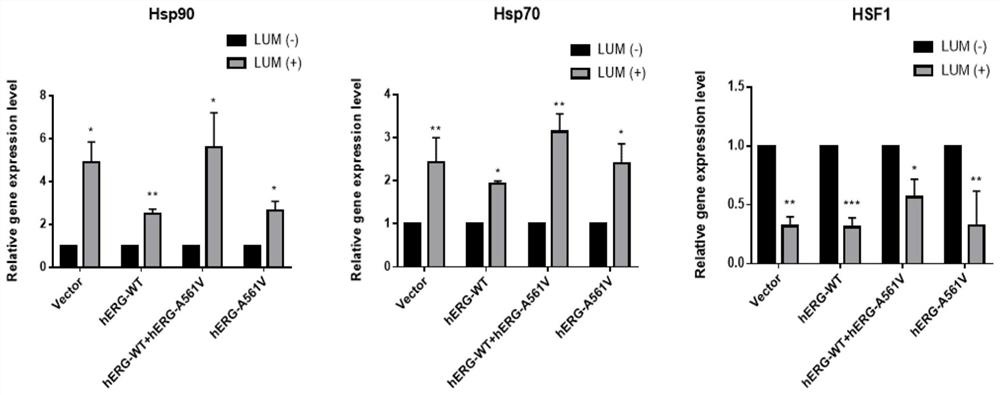
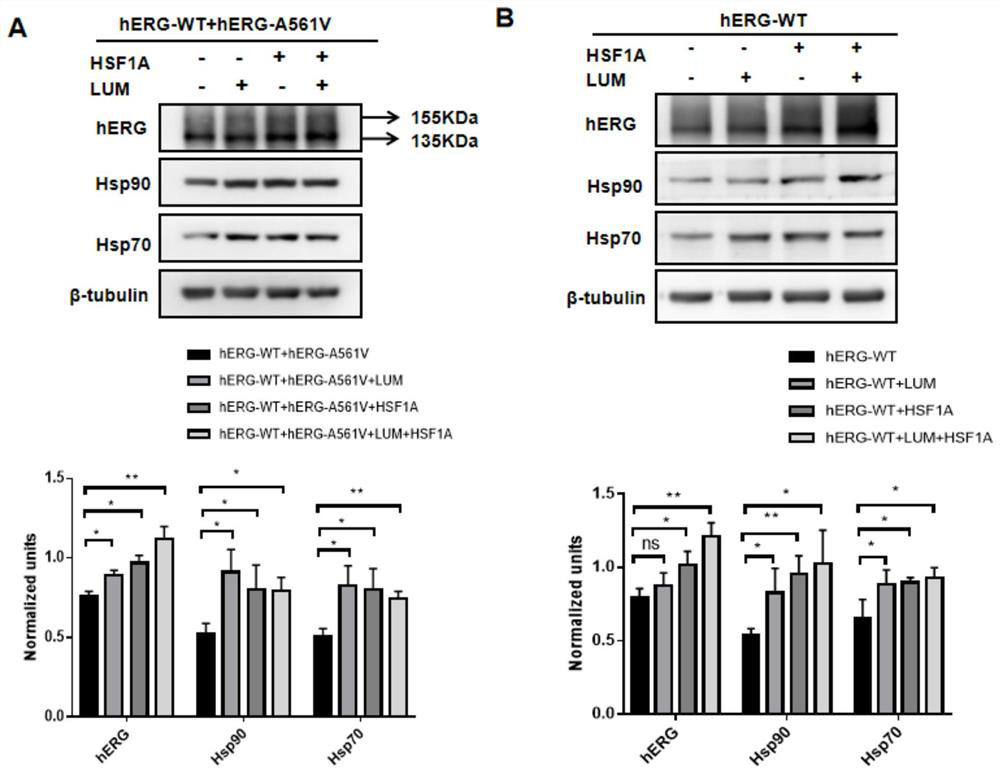
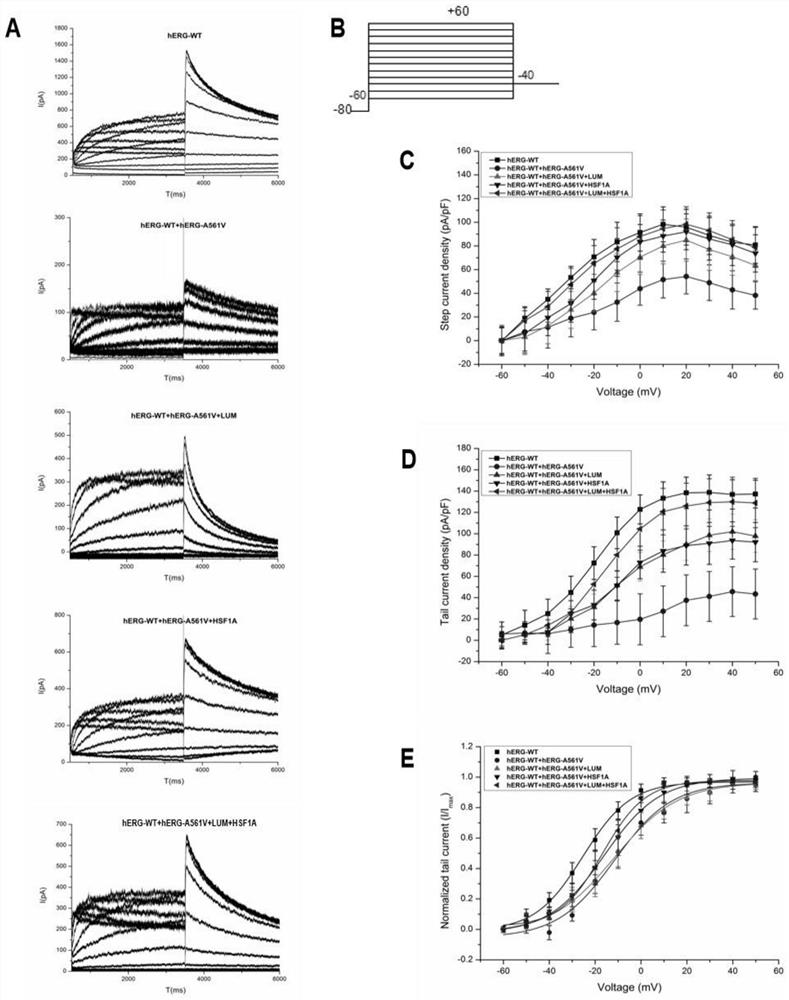
[0021] Cell culture and construction of hERG translocation disorder model: by transient transfection, pcDNA3-Mock, pcDNA3-HERG, pcDNA3-HERG / pcDNA3-HERG-A561V, pcDNA3-HERG-A561V plasmids were transfected to produce control, wild, heterozygous and HEK293 cell line with mutated hERG. The above cells were cultured in DMEM (high glucose) containing 10% fetal bovine serum, and maintained in an incubator environment at 37° C. containing 5% carbon dioxide. Cells were harvested from the dish, resuspended as single cells and cultured in small dishes of fresh MEM for at least 4 hours before use.
[0022] Quantitative RT-PCR: Cells before and after drug treatment were lysed with TransZol Up (whole gold) and total RNA was extracted, reverse-transcribed into cDNA, and 1 μg was used as a template, configured according to the 2×T5 Fast qPCR Mix (Qingke Bio) The 20 μl reaction system was used to detect the expression of the target gene, and SYBR Green I was used as the expression signal. Duri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com