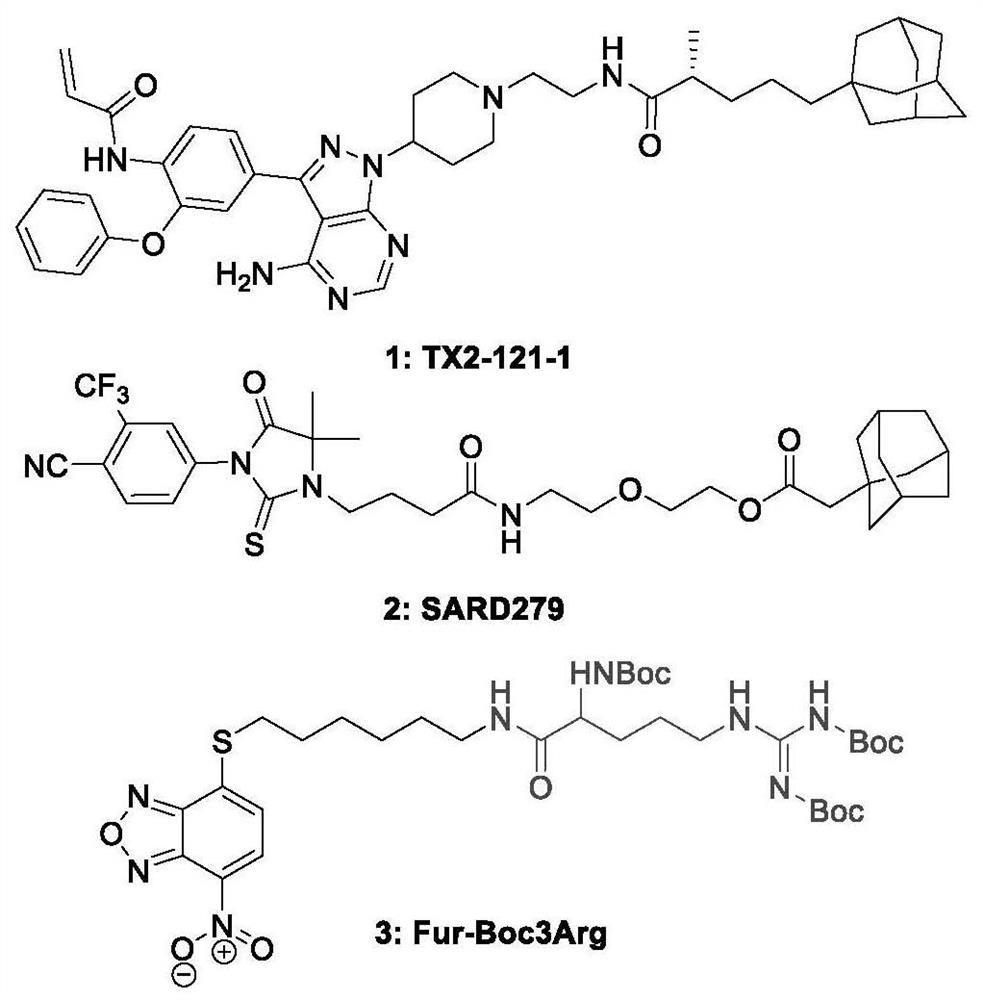
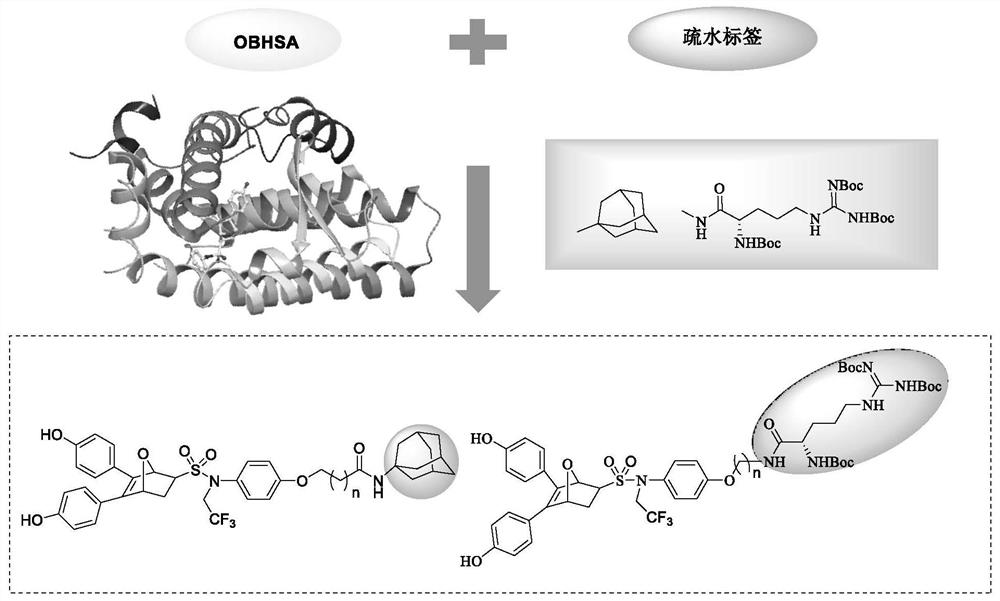
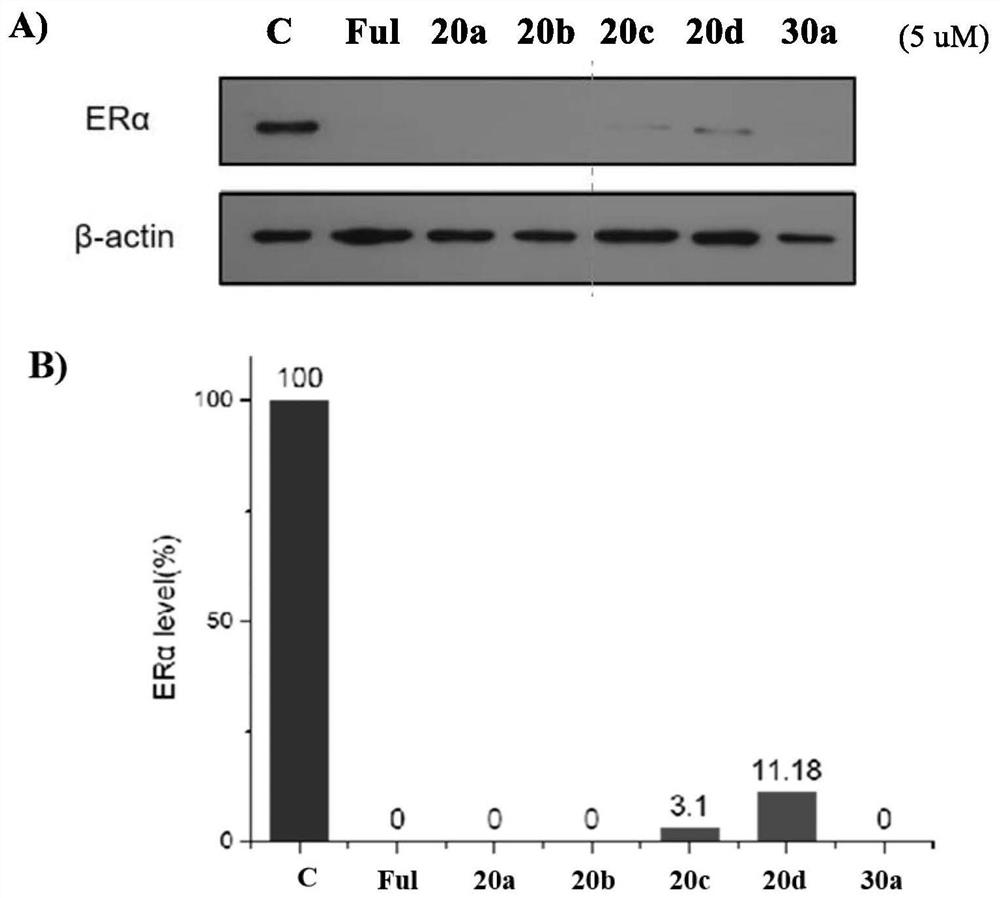
Hydrophobic label-containing oxygen-bridged bicyclo-heptylene sulfonamide compound and application thereof
A cycloheptene sulfonamide and compound technology, applied in the field of medicine, can solve the problems of unsatisfactory molecular rigidity and water solubility, difficult to obtain a patterned design strategy, poor oral absorption and membrane permeability, etc., and achieve good estrogen Effects of receptor down-regulation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: (2S)-2-amino-N-(3-(4-((5,6-bis(4-hydroxyphenyl)-N-(2,2,2-trifluoroethyl)- 7) Preparation of -[2.2.1]hept-5-ene)-2-sulfonamide (phenoxy)propyl)-5-guanidine pentaneamide derivative (30a):
[0071]
[0072] Compound 29 (74.62mg, 0.126mmol) and compound 28a (85.95mg, 0.15mmol) were placed in a 25mL single-necked bottle, dissolved in 2mL of THF, triethylamine (15.21mg, 0.15mmol) was added, and reacted for 2h, After TLC confirms that the reaction is complete, it is extracted with ethyl acetate. After the organic phase is concentrated and spin-dried, it is dried with anhydrous sodium sulfate, and is separated and purified by silica gel column chromatography (the eluent ratio is v 二氯甲烷 / v 甲醇 =120:1-30:1), to obtain 108 mg of yellow solid compound 30a, with a yield of 77%. 1 HNMR (400MHz, MeOD-d 6)δ7.19(d, J=8.8Hz, 2H), 7.12(t, J=8.4Hz, 4H), 6.78(t, J=9.1Hz, 4H), 6.69(d, J=8.7Hz, 2H) ,5.45(s,1H),5.29(d,J=4.2Hz,1H),4.40(q,J=8.4Hz,2H),3.97(t,J=6.1Hz,3H),3.84(td,J...
Embodiment 2
[0073] Example 2: (2R)-2-amino-N-(6-(4-((5,6-bis(4-hydroxyphenyl)-N-(2,2,2-trifluoroethyl)- 7) Preparation of -[2.2.1]hept-5-ene)-2-sulfonamide (phenoxy)hexyl)-5-guanidine pentaneamide derivative (30b):
[0074]
[0075] Compound 29 (40mg, 0.068mmol) and compound 28b (57.3mg, 0.10mmol) were placed in a 25mL single-necked bottle, dissolved in 2mL of THF, added triethylamine (8.2mg, 0.08mmol), reacted for 2h, TLC After confirming that the reaction was complete, it was extracted with ethyl acetate, the organic phase was concentrated and spin-dried, dried with anhydrous sodium sulfate, separated and purified by silica gel column chromatography (the eluent ratio was v 二氯甲烷 / v 甲醇 =100:1-15:1), 47 mg of light yellow solid compound 30b was obtained, and the yield was 50%. The H NMR spectrum of point 1: 1 H NMR (400MHz, Acetone-d 6 )δ7.55(s,1H),7.33(d,J=2.2Hz,1H),7.30(d,J=2.2Hz,1H),7.26(d,J=4.3Hz,4H),7.20(dd, J=8.5,4.3Hz,5H),6.88(d,J=1.2Hz,1H),6.86(d,J=1.2Hz,1H),6.83(d,J=2.7Hz,...
Embodiment 3
[0076] Example 3: tert-butyl ((2S)-1-((3-(4-((1R,4R)-5,6-bis(4-hydroxyphenyl)-N-(2,2,2- Trifluoroethyl)-7-oxabicyclo[2.2.1]hept-5-ene)-2-sulfonyl)phenoxy)propyl)amino)-1-oxo-3-phenylpropan-2- base) carbamate (30c):
[0077]
[0078] Compound 29 (40mg, 0.068mmol) and compound 28c (32mg, 0.10mmol) were placed in a 25mL single-necked bottle, dissolved in 2mL of THF, triethylamine (8.2mg, 0.08mmol) was added, reacted for 2h, and confirmed by TLC After the reaction was complete, it was extracted with ethyl acetate, the organic phase was concentrated and spin-dried, dried with anhydrous sodium sulfate, separated and purified by silica gel column chromatography (the eluent ratio was v 二氯甲烷 / v 甲醇 =100:1-15:1), 47 mg of light yellow solid compound 30c was obtained with a yield of 86%. The H NMR spectrum of point 1: 1 H NMR (400MHz, Acetone-d 6 )δ7.55(s,1H),7.33(d,J=2.2Hz,1H),7.30(d,J=2.2Hz,1H),7.26(d,J=4.3Hz,4H),7.20(dd, J=8.5,4.3Hz,5H),6.88(d,J=1.2Hz,1H),6.86(d,J=1.2Hz,1H),6....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com